II International Symposium on Carbon for Catalysis ABSTRACTS
II International Symposium on Carbon for Catalysis ABSTRACTS
II International Symposium on Carbon for Catalysis ABSTRACTS
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
PP-I-11<br />
We suppose that the active OCP are the three-dimensi<strong>on</strong>al polymer presented by<br />
graphitized and c<strong>on</strong>densed aromatic structures cross-linked by alkyl or sulfur bridges. The<br />
quin<strong>on</strong>e, C=S, lact<strong>on</strong>e, carboxyl, and alkyl groups in oxidative c<strong>on</strong>densati<strong>on</strong> products were<br />
detected by DRIFTS. There are probably the terminal substituents of polynuclear structures.<br />
The sulfate groups were found at the surface of carb<strong>on</strong>ized alunima.<br />
The catalytic properties of OCP depends <strong>on</strong> the oxygen functi<strong>on</strong>al surface groups<br />
compositi<strong>on</strong>. It is not revealed lact<strong>on</strong>e groups in deactivated coke.<br />
The coke morphology <strong>on</strong> the surface of silica is significantly different from <strong>on</strong>e <strong>on</strong><br />
alumina or Si - Al oxides. Aggregated clusters of OCP were <strong>for</strong>med <strong>on</strong> silica, but total<br />
coverage of coke was <strong>for</strong>med <strong>on</strong> alumina surface.<br />
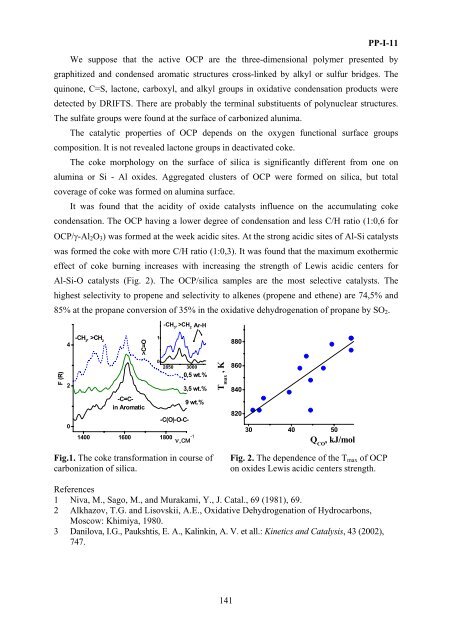
It was found that the acidity of oxide catalysts influence <strong>on</strong> the accumulating coke<br />
c<strong>on</strong>densati<strong>on</strong>. The OCP having a lower degree of c<strong>on</strong>densati<strong>on</strong> and less C/H ratio (1:0,6 <strong>for</strong><br />
OCP/γ-Al 2 O 3 ) was <strong>for</strong>med at the week acidic sites. At the str<strong>on</strong>g acidic sites of Al-Si catalysts<br />
was <strong>for</strong>med the coke with more C/H ratio (1:0,3). It was found that the maximum exothermic<br />
effect of coke burning increases with increasing the strength of Lewis acidic centers <strong>for</strong><br />
Al-Si-O catalysts (Fig. 2). The OCP/silica samples are the most selective catalysts. The<br />
highest selectivity to propene and selectivity to alkenes (propene and ethene) are 74,5% and<br />
85% at the propane c<strong>on</strong>versi<strong>on</strong> of 35% in the oxidative dehydrogenati<strong>on</strong> of propane by SО 2 .<br />
-CH 3<br />
, >CH 2 Ar-H<br />
4<br />
-CH 3<br />
, >CH 2<br />
>C=O<br />
1<br />
880<br />
F (R)<br />
2<br />
0<br />
-C=Cin<br />
Aromatic<br />
1400 1600 1800<br />
0<br />
2850 3000<br />
-C(O)-O-C-<br />
0,5 wt.%<br />
3,5 wt.%<br />
ν,см -1<br />
9 wt.%<br />
T max<br />
, K<br />
860<br />
840<br />
820<br />
30 40 50<br />
Q CO<br />
, kJ/mol<br />
Fig.1. The coke trans<strong>for</strong>mati<strong>on</strong> in course of<br />
carb<strong>on</strong>izati<strong>on</strong> of silica.<br />
Fig. 2. The dependence of the T max of OCP<br />
<strong>on</strong> oxides Lewis acidic centers strength.<br />
References<br />
1 Niva, M., Sago, M., and Murakami, Y., J. Catal., 69 (1981), 69.<br />
2 Alkhazov, T.G. and Lisovskii, A.E., Oxidative Dehydrogenati<strong>on</strong> of Hydrocarb<strong>on</strong>s,<br />
Moscow: Khimiya, 1980.<br />
3 Danilova, I.G., Paukshtis, E. A., Kalinkin, A. V. et all.: Kinetics and <strong>Catalysis</strong>, 43 (2002),<br />
747.<br />
141