II International Symposium on Carbon for Catalysis ABSTRACTS
II International Symposium on Carbon for Catalysis ABSTRACTS
II International Symposium on Carbon for Catalysis ABSTRACTS
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
PP-<str<strong>on</strong>g>II</str<strong>on</strong>g>-17<br />
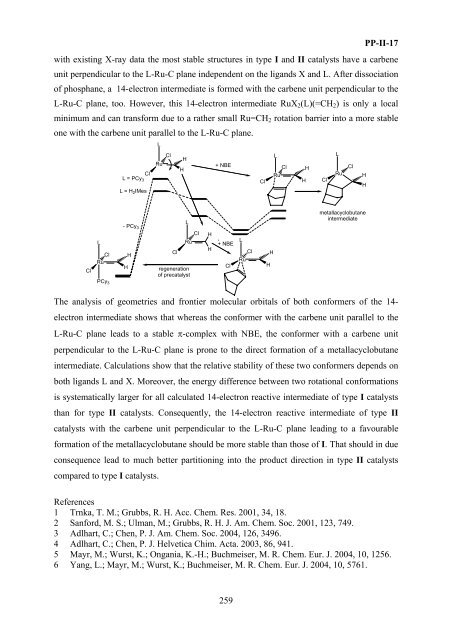
with existing X-ray data the most stable structures in type I and <str<strong>on</strong>g>II</str<strong>on</strong>g> catalysts have a carbene<br />
unit perpendicular to the L-Ru-C plane independent <strong>on</strong> the ligands X and L. After dissociati<strong>on</strong><br />
of phosphane, a 14-electr<strong>on</strong> intermediate is <strong>for</strong>med with the carbene unit perpendicular to the<br />
L-Ru-C plane, too. However, this 14-electr<strong>on</strong> intermediate RuX 2 (L)(=CH 2 ) is <strong>on</strong>ly a local<br />
minimum and can trans<strong>for</strong>m due to a rather small Ru=CH 2 rotati<strong>on</strong> barrier into a more stable<br />
<strong>on</strong>e with the carbene unit parallel to the L-Ru-C plane.<br />
Cl<br />
L<br />
Cl<br />
Ru<br />
PCy 3<br />
+ NBE<br />
Cl<br />
H<br />
Ru<br />
H<br />
Cl<br />
L = PCy 3<br />
- PCy 3<br />
L<br />
L = H 2 IMes<br />
H<br />
H<br />
L<br />
Cl<br />
Ru<br />
regenerati<strong>on</strong><br />
of precatalyst<br />
Cl<br />
H<br />
H<br />
+ NBE<br />
Cl<br />
L<br />
Ru<br />
Cl<br />
Cl<br />
H<br />
H<br />
L<br />
Ru<br />
Cl<br />
H<br />
H<br />
Cl<br />
L<br />
Ru<br />
Cl<br />
H<br />
H<br />
metallacyclobutane<br />
intermediate<br />
The analysis of geometries and fr<strong>on</strong>tier molecular orbitals of both c<strong>on</strong><strong>for</strong>mers of the 14-<br />
electr<strong>on</strong> intermediate shows that whereas the c<strong>on</strong><strong>for</strong>mer with the carbene unit parallel to the<br />
L-Ru-C plane leads to a stable π-complex with NBE, the c<strong>on</strong><strong>for</strong>mer with a carbene unit<br />
perpendicular to the L-Ru-C plane is pr<strong>on</strong>e to the direct <strong>for</strong>mati<strong>on</strong> of a metallacyclobutane<br />
intermediate. Calculati<strong>on</strong>s show that the relative stability of these two c<strong>on</strong><strong>for</strong>mers depends <strong>on</strong><br />
both ligands L and X. Moreover, the energy difference between two rotati<strong>on</strong>al c<strong>on</strong><strong>for</strong>mati<strong>on</strong>s<br />
is systematically larger <strong>for</strong> all calculated 14-electr<strong>on</strong> reactive intermediate of type I catalysts<br />
than <strong>for</strong> type <str<strong>on</strong>g>II</str<strong>on</strong>g> catalysts. C<strong>on</strong>sequently, the 14-electr<strong>on</strong> reactive intermediate of type <str<strong>on</strong>g>II</str<strong>on</strong>g><br />
catalysts with the carbene unit perpendicular to the L-Ru-C plane leading to a favourable<br />
<strong>for</strong>mati<strong>on</strong> of the metallacyclobutane should be more stable than those of I. That should in due<br />
c<strong>on</strong>sequence lead to much better partiti<strong>on</strong>ing into the product directi<strong>on</strong> in type <str<strong>on</strong>g>II</str<strong>on</strong>g> catalysts<br />
compared to type I catalysts.<br />
References<br />
1 Trnka, T. M.; Grubbs, R. H. Acc. Chem. Res. 2001, 34, 18.<br />
2 San<strong>for</strong>d, M. S.; Ulman, M.; Grubbs, R. H. J. Am. Chem. Soc. 2001, 123, 749.<br />
3 Adlhart, C.; Chen, P. J. Am. Chem. Soc. 2004, 126, 3496.<br />
4 Adlhart, C.; Chen, P. J. Helvetica Chim. Acta. 2003, 86, 941.<br />
5 Mayr, M.; Wurst, K.; Ongania, K.-H.; Buchmeiser, M. R. Chem. Eur. J. 2004, 10, 1256.<br />
6 Yang, L.; Mayr, M.; Wurst, K.; Buchmeiser, M. R. Chem. Eur. J. 2004, 10, 5761.<br />
259