II International Symposium on Carbon for Catalysis ABSTRACTS
II International Symposium on Carbon for Catalysis ABSTRACTS
II International Symposium on Carbon for Catalysis ABSTRACTS
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
PP-I-15<br />
Titania was prepared by hydrolysis of TiCl 4 soluti<strong>on</strong> using polyethylene imine (PEI) when<br />
deposited <strong>on</strong>to the CNF/CF [4]. Gold colloids were prepared from a HAuCl 4 soluti<strong>on</strong> and added<br />
dropwise to the suspended TiO 2 /CNF/CF to produce the catalyst [5,6]. The catalysts are being<br />
characterised by several methods such as BET, XRD, TPO, SEM and TEM.<br />
Productivity C/gcat] [g<br />
80<br />
70<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
Lower space<br />
velocity<br />
Higher space<br />
velocity<br />
0.00 1.00 2.00 3.00 4.00 5.00<br />
Time [hour]<br />
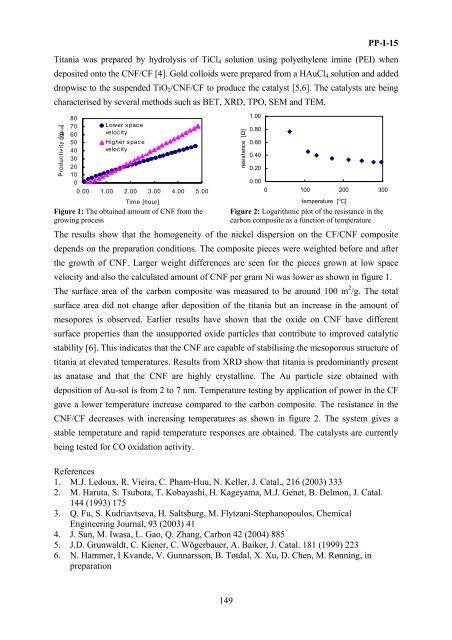
Figure 1: The obtained amount of CNF from the<br />
growing process<br />
resistance [Ω]<br />
1.00<br />
0.80<br />
0.60<br />
0.40<br />
0.20<br />
0.00<br />
0 100 200 300<br />
temperature [ o C]<br />
Figure 2: Logarithmic plot of the resistance in the<br />
carb<strong>on</strong> composite as a functi<strong>on</strong> of temperature<br />
The results show that the homogeneity of the nickel dispersi<strong>on</strong> <strong>on</strong> the CF/CNF composite<br />
depends <strong>on</strong> the preparati<strong>on</strong> c<strong>on</strong>diti<strong>on</strong>s. The composite pieces were weighted be<strong>for</strong>e and after<br />
the growth of CNF. Larger weight differences are seen <strong>for</strong> the pieces grown at low space<br />
velocity and also the calculated amount of CNF per gram Ni was lower as shown in figure 1.<br />
The surface area of the carb<strong>on</strong> composite was measured to be around 100 m 2 /g. The total<br />
surface area did not change after depositi<strong>on</strong> of the titania but an increase in the amount of<br />
mesopores is observed. Earlier results have shown that the oxide <strong>on</strong> CNF have different<br />
surface properties than the unsupported oxide particles that c<strong>on</strong>tribute to improved catalytic<br />
stability [6]. This indicates that the CNF are capable of stabilising the mesoporous structure of<br />
titania at elevated temperatures. Results from XRD show that titania is predominantly present<br />
as anatase and that the CNF are highly crystalline. The Au particle size obtained with<br />
depositi<strong>on</strong> of Au-sol is from 2 to 7 nm. Temperature testing by applicati<strong>on</strong> of power in the CF<br />
gave a lower temperature increase compared to the carb<strong>on</strong> composite. The resistance in the<br />
CNF/CF decreases with increasing temperatures as shown in figure 2. The system gives a<br />
stable temperature and rapid temperature resp<strong>on</strong>ses are obtained. The catalysts are currently<br />
being tested <strong>for</strong> CO oxidati<strong>on</strong> activity.<br />
References<br />
1. M.J. Ledoux, R. Vieira, C. Pham-Huu, N. Keller, J. Catal., 216 (2003) 333<br />
2. M. Haruta, S. Tsubota, T. Kobayashi, H. Kageyama, M.J. Genet, B. Delm<strong>on</strong>, J. Catal.<br />
144 (1993) 175<br />
3. Q. Fu, S. Kudriavtseva, H. Saltsburg, M. Flytzani-Stephanopoulos, Chemical<br />
Engineering Journal, 93 (2003) 41<br />
4. J. Sun, M. Iwasa, L. Gao, Q. Zhang, Carb<strong>on</strong> 42 (2004) 885<br />
5. J.D. Grunwaldt, C. Kiener, C. Wögerbauer, A. Baiker, J. Catal. 181 (1999) 223<br />
6. N. Hammer, I Kvande, V. Gunnarss<strong>on</strong>, B. Tøtdal, X. Xu, D. Chen, M. Rønning, in<br />
preparati<strong>on</strong><br />
149