II International Symposium on Carbon for Catalysis ABSTRACTS
II International Symposium on Carbon for Catalysis ABSTRACTS
II International Symposium on Carbon for Catalysis ABSTRACTS
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
PP-I-42<br />
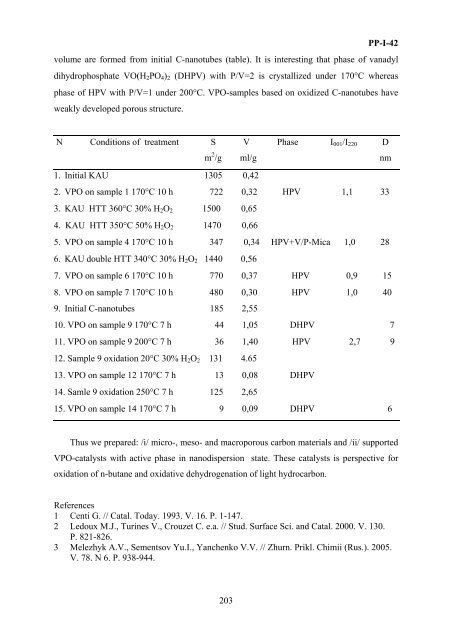
volume are <strong>for</strong>med from initial C-nanotubes (table). It is interesting that phase of vanadyl<br />
dihydrophosphate VO(H 2 PO 4 ) 2 (DHPV) with P/V=2 is crystallized under 170°C whereas<br />
phase of HPV with P/V=1 under 200°C. VPO-samples based <strong>on</strong> oxidized C-nanotubes have<br />
weakly developed porous structure.<br />
N C<strong>on</strong>diti<strong>on</strong>s of treatment S V Phase I 001 /I 220 D<br />
m 2 /g ml/g nm<br />
1. Initial KAU 1305 0,42<br />
2. VPO <strong>on</strong> sample 1 170°C 10 h 722 0,32 HPV 1,1 33<br />
3. KAU HTT 360°C 30% H 2 O 2 1500 0,65<br />
4. KAU HTT 350°C 50% H 2 O 2 1470 0,66<br />
5. VPO <strong>on</strong> sample 4 170°C 10 h 347 0,34 HPV+V/P-Mica 1,0 28<br />
6. KAU double HTT 340°C 30% H 2 O 2 1440 0,56<br />
7. VPO <strong>on</strong> sample 6 170°C 10 h 770 0,37 HPV 0,9 15<br />
8. VPO <strong>on</strong> sample 7 170°C 10 h 480 0,30 HPV 1,0 40<br />
9. Initial C-nanotubes 185 2,55<br />
10. VPO <strong>on</strong> sample 9 170°C 7 h 44 1,05 DHPV 7<br />
11. VPO <strong>on</strong> sample 9 200°C 7 h 36 1,40 HPV 2,7 9<br />
12. Sample 9 oxidati<strong>on</strong> 20°C 30% H 2 O 2 131 4.65<br />
13. VPO <strong>on</strong> sample 12 170°C 7 h 13 0,08 DHPV<br />
14. Samle 9 oxidati<strong>on</strong> 250°C 7 h 125 2,65<br />
15. VPO <strong>on</strong> sample 14 170°C 7 h 9 0,09 DHPV 6<br />
Thus we prepared: /i/ micro-, meso- and macroporous carb<strong>on</strong> materials and /ii/ supported<br />
VPO-catalysts with active phase in nanodispersi<strong>on</strong> state. These catalysts is perspective <strong>for</strong><br />
oxidati<strong>on</strong> of n-butane and oxidative dehydrogenati<strong>on</strong> of light hydrocarb<strong>on</strong>.<br />
References<br />
1 Centi G. // Catal. Today. 1993. V. 16. P. 1-147.<br />
2 Ledoux M.J., Turines V., Crouzet C. e.a. // Stud. Surface Sci. and Catal. 2000. V. 130.<br />
P. 821-826.<br />
3 Melezhyk A.V., Sementsov Yu.I., Yanchenko V.V. // Zhurn. Prikl. Chimii (Rus.). 2005.<br />
V. 78. N 6. P. 938-944.<br />
203