II International Symposium on Carbon for Catalysis ABSTRACTS
II International Symposium on Carbon for Catalysis ABSTRACTS
II International Symposium on Carbon for Catalysis ABSTRACTS
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
OP-I-2<br />
loading. It is also interesting to observe that the particle sizes <strong>on</strong>ly increase very slightly with<br />
the metal loading.<br />
TEM characterizati<strong>on</strong> dem<strong>on</strong>strates that all the cobalt is deposited <strong>on</strong> the fibres, free of<br />
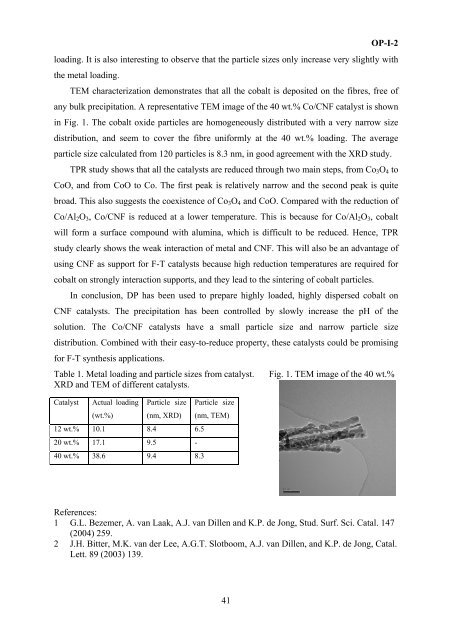
any bulk precipitati<strong>on</strong>. A representative TEM image of the 40 wt.% Co/CNF catalyst is shown<br />
in Fig. 1. The cobalt oxide particles are homogeneously distributed with a very narrow size<br />
distributi<strong>on</strong>, and seem to cover the fibre uni<strong>for</strong>mly at the 40 wt.% loading. The average<br />
particle size calculated from 120 particles is 8.3 nm, in good agreement with the XRD study.<br />
TPR study shows that all the catalysts are reduced through two main steps, from Co 3 O 4 to<br />
CoO, and from CoO to Co. The first peak is relatively narrow and the sec<strong>on</strong>d peak is quite<br />
broad. This also suggests the coexistence of Co 3 O 4 and CoO. Compared with the reducti<strong>on</strong> of<br />
Co/Al 2 O 3 , Co/CNF is reduced at a lower temperature. This is because <strong>for</strong> Co/Al 2 O 3 , cobalt<br />
will <strong>for</strong>m a surface compound with alumina, which is difficult to be reduced. Hence, TPR<br />
study clearly shows the weak interacti<strong>on</strong> of metal and CNF. This will also be an advantage of<br />
using CNF as support <strong>for</strong> F-T catalysts because high reducti<strong>on</strong> temperatures are required <strong>for</strong><br />
cobalt <strong>on</strong> str<strong>on</strong>gly interacti<strong>on</strong> supports, and they lead to the sintering of cobalt particles.<br />
In c<strong>on</strong>clusi<strong>on</strong>, DP has been used to prepare highly loaded, highly dispersed cobalt <strong>on</strong><br />
CNF catalysts. The precipitati<strong>on</strong> has been c<strong>on</strong>trolled by slowly increase the pH of the<br />
soluti<strong>on</strong>. The Co/CNF catalysts have a small particle size and narrow particle size<br />
distributi<strong>on</strong>. Combined with their easy-to-reduce property, these catalysts could be promising<br />
<strong>for</strong> F-T synthesis applicati<strong>on</strong>s.<br />
Table 1. Metal loading and particle sizes from catalyst. Fig. 1. TEM image of the 40 wt.%<br />
XRD and TEM of different catalysts.<br />
Catalyst Actual loading Particle size Particle size<br />
(wt.%) (nm, XRD) (nm, TEM)<br />
12 wt.% 10.1 8.4 6.5<br />
20 wt.% 17.1 9.5 -<br />
40 wt.% 38.6 9.4 8.3<br />
References:<br />
1 G.L. Bezemer, A. van Laak, A.J. van Dillen and K.P. de J<strong>on</strong>g, Stud. Surf. Sci. Catal. 147<br />
(2004) 259.<br />
2 J.H. Bitter, M.K. van der Lee, A.G.T. Slotboom, A.J. van Dillen, and K.P. de J<strong>on</strong>g, Catal.<br />
Lett. 89 (2003) 139.<br />
41