- Page 1 and 2:
Boreskov Institute of Catalysis of
- Page 3:
ORGANIZING COMMITTEE Chairman: Vlad
- Page 7 and 8:
CATALYSTS ON CARBON MATERIALS BASIS
- Page 9 and 10:
PREPARATION OF CARBON FILMS ON CERA
- Page 11 and 12:
PL-2 polymer gas separation membran
- Page 13 and 14:
CHARACTERIZATION OF POROUS STRUCTUR
- Page 15 and 16:
ROLE OF CARBON POROSITY AND FUNCTIO
- Page 17:
KEYNOTE LECTURES
- Page 20 and 21:
KL-1 governed by the equation of th
- Page 22 and 23:
KL-2 NITROGEN DOPED CARBON NANOFIBE
- Page 24 and 25:
KL-3 CARBON BASED STRUCTURED CATALY
- Page 26 and 27:
KL-4 The strong mesoporous carbons
- Page 28 and 29:
KL-5 NANOSTRUCTURED CARBONS FOR THE
- Page 30 and 31:
KL-6 THEORY OF MECHANICAL BEHAVIOR
- Page 32 and 33:
KL-7 MOLECULAR STRUCTURE EFFECT OF
- Page 34 and 35:
Presentation of FGUP “ENPO Neorga
- Page 37 and 38:
OP-I-1 COBALT ON CARBON NANOFIBER C
- Page 39 and 40:
OP-I-1 from 41 to 23 %, while the C
- Page 41 and 42:
OP-I-2 loading. It is also interest
- Page 43 and 44:
OP-I-3 5 nm 50 nm Figure 1: PtRu/MW
- Page 45 and 46:
OP-I-4 As it can be seen, potassium
- Page 47 and 48:
OP-I-5 [A] ⇔ A+[ ] (5) Kinetic an
- Page 49 and 50:
Results and discussion OP-I-6 The T
- Page 51 and 52:
OP-I-7 product selectivity). The an
- Page 53 and 54:
OP-I-8 After introduction of the me
- Page 55 and 56:
OP-I-9 Hydrogenations were carried
- Page 57 and 58:
OP-I-10 activated with CO 2 . In al
- Page 59 and 60:
OP-I-11 from Jaroszów deposit, Low
- Page 61 and 62:
OP-I-12 The carbon matrix avoids th
- Page 63 and 64:
OP-I-13 • thermal destruction of
- Page 65 and 66:
OP-I-14 hexanol (AA) was anchored t
- Page 67 and 68:
OP-I-15 removing the physically ads
- Page 69 and 70:
OP-I-16 surface concentration of HE
- Page 71 and 72:
Our analysis of the chemical activi
- Page 73 and 74:
OP-I-18 running. The TEM reveals th
- Page 75 and 76:
OP-I-19 explore the role of CNF sur
- Page 77 and 78:
pH 10 8 6 4 2 0 2 4 6 8 10 ml HCl a
- Page 79 and 80:
OP-I-21 resorcinol to catalyst mola
- Page 81 and 82:
OP-I-22 AS in the catalysts examine
- Page 83 and 84:
OP-I-23 polyfunctional surface cove
- Page 85 and 86:
OP-I-24 as sibunite and nanofibrous
- Page 87 and 88:
OP-I-25 is a consequence of the mac
- Page 89 and 90:
OP-I-27 THE CARBON FILMS ON Pt(111)
- Page 91 and 92:
OP-I-28 LASER RAMAN MICRO-SPECTROSC
- Page 93 and 94:
OP-I-29 ELECTROCATALYTIC PROPERTIES
- Page 95 and 96:
OP-II-1 SYNTHESIS
- Page 97 and 98:
OP-II-2 SYNTHESIS,
- Page 99 and 100:
TOWARDS LARGE SCALE PRODUCTION OF C
- Page 101 and 102:
CO-CARBONIZATION OF POLYMERS - NEW
- Page 103 and 104:
OP-II-5 3. Results
- Page 105 and 106:
OP-II-6 pore size
- Page 107 and 108:
OP-II-7 their adva
- Page 109 and 110:
OP-II-8 effect, el
- Page 111 and 112:
OP-II-9 Therefore,
- Page 113 and 114:
OP-II-10 The dehyd
- Page 115:
OP-II-11 a result
- Page 119 and 120:
INVESTIGATION OF THE ELECTROCHEMICA
- Page 121 and 122:
SURFACE ELECTROCHEMISTRY OF CARBON
- Page 123 and 124:
TEMPLATED SYNTHESIS OF NOBLE METAL
- Page 125 and 126:
PROPERTIES AND STRUCTURE OF SORBENT
- Page 127 and 128:
CATALYSIS DURING SULPHUR COALS PROC
- Page 129 and 130:
ORDERED MESOPOROUS CARBONS SYNTHESI
- Page 131 and 132:
PP-I-7 INFLUENCE OF FUNCTIONALIZATI
- Page 133 and 134:
PP-I-8 AROMATIZATION OF 5-PHENYL-PI
- Page 135 and 136:
N 2 O CONVERSION USING BINARY MIXTU
- Page 137 and 138:
PP-I-9 [8] F. Gonçalves, G.E. Marn
- Page 139 and 140: PP-I-10 The Table 1 demonstrates th
- Page 141 and 142: PP-I-11 We suppose that the active
- Page 143 and 144: PP-I-12 Activation with steam promo
- Page 145 and 146: PP-I-13 separation of bundles forme
- Page 147 and 148: PP-I-14 temperature at which the ma
- Page 149 and 150: PP-I-15 Titania was prepared by hyd
- Page 151 and 152: PP-I-16 A B Figure 1. SEM images co
- Page 153 and 154: PP-I-17 the carbon matrix has been
- Page 155 and 156: PP-I-18 0.9 0.8 0.7 0.6 lg (ΔV/ΔR
- Page 157 and 158: PP-I-19 Electron-microscopic studie
- Page 159 and 160: PP-I-20 time on stream increased fr
- Page 161 and 162: CARBON-SUPPORTED 12-MOLYBDOPHOSPHOR
- Page 163 and 164: PP-I-23 CARBON SUPPORTS FOR IMMOBIL
- Page 165 and 166: PP-I-24 example, in the polymer ele
- Page 167 and 168: PP-I-25 of microporous structure wa
- Page 169 and 170: PP-I-26 contain at first. Non-oxida
- Page 171 and 172: PP-I-27 this reaction and that the
- Page 173 and 174: CONTROLLING DIAMETER AND PRESENCE O
- Page 175 and 176: INFLUENCE OF GAS OXIDATIVE TREATMEN
- Page 177 and 178: PP-I-30 EXPERIMENTAL VALUES OF THE
- Page 179 and 180: PP-I-30 Acknowledgements The author
- Page 181 and 182: PP-I-31 suspensions of UFD had low
- Page 183 and 184: PP-I-32 It was detected, that precu
- Page 185 and 186: PP-I-33 consistence of the process,
- Page 187 and 188: PP-I-34 practically identical [2],
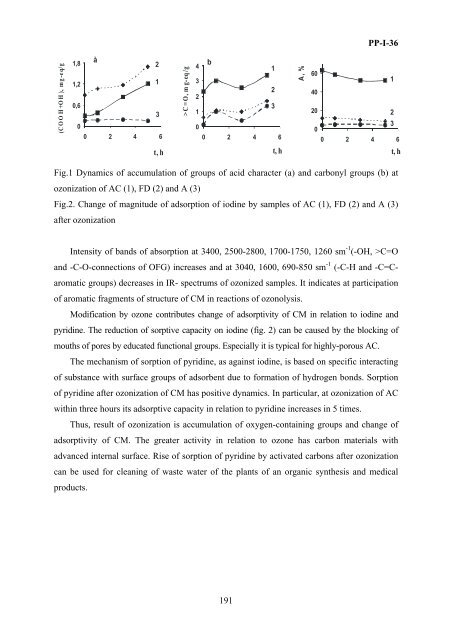
- Page 189: PP-I-35 • surface porosity (P 245
- Page 193 and 194: PP-I-37 As one can see in the table
- Page 195 and 196: PP-I-38 hydrogenation (61 o and 65
- Page 197 and 198: PP-I-39 Ensembles №№ 31, 45, 55
- Page 199 and 200: PP-I-40 The pore structure of origi
- Page 201 and 202: PP-I-41 Sr 2+ and Ba 2+ ions are in
- Page 203 and 204: PP-I-42 volume are formed from init
- Page 205 and 206: PP-I-43 30 wt% Pt-15 wt% Ru/NCM cat
- Page 207 and 208: PP-I-44 Figure 1. From left to righ
- Page 209 and 210: PP-I-45 Table 1: Characteristics of
- Page 211 and 212: PP-I-45 The search for alternative
- Page 213 and 214: PP-I-46 zeroth moment expression, w
- Page 215 and 216: PP-I-47 It was revealed, that the i
- Page 217 and 218: PP-I-48 Table 1 Characteristics of
- Page 219 and 220: PP-I-49 4-Carboxybenzaldehyde (4-CB
- Page 221 and 222: PP-I-50 Computational details First
- Page 223 and 224: PP-I-51 The result showed that surf
- Page 225 and 226: PP-II-1 COOH COO(C
- Page 227 and 228: PP-II-2 The result
- Page 229 and 230: PP-II-3 prepared,
- Page 231 and 232: PP-II-4 CNF from b
- Page 233 and 234: PP-II-5 support; t
- Page 235 and 236: PP-II-6 The main p
- Page 237 and 238: PP-II-7 porous net
- Page 239 and 240: PP-II-8 It is note
- Page 241 and 242:
PP-II-9 selective
- Page 243 and 244:
STABILITY OF A CARBON CATALYST FLUI
- Page 245 and 246:
PP-II-11 CARBON FI
- Page 247 and 248:
PP-II-12 CATALYTIC
- Page 249 and 250:
THE SORBENTS FOR AIR CLEANING PREPA
- Page 251 and 252:
MACRO-SHAPING OF CARBON NANOFIBERS
- Page 253 and 254:
PP-II-15 MESOPOROU
- Page 255 and 256:
PP-II-16 EVALUATIO
- Page 257 and 258:
PP-II-16 As it wou
- Page 259 and 260:
PP-II-17 with exis
- Page 261 and 262:
PP-II-18 Thus, the
- Page 263 and 264:
PP-II-19 (NaOH, Cs
- Page 265 and 266:
PP-II-20 MEDICAL C
- Page 267 and 268:
PP-II-21 Pt /AC an
- Page 269 and 270:
PP-II-22 Prelimina
- Page 271 and 272:
FACTORS DETERMINING THE CATALYTIC P
- Page 273 and 274:
NANOPOROUS CARBON MATERIALS AS EFFE
- Page 275 and 276:
CARBON NANOSTRUCTURAL MATERIALS PRO
- Page 277 and 278:
GROWTH OF CARBON NANOFIBERS ON META
- Page 279 and 280:
ACTIVE CARBON AS SUPPORT FOR IMMOBI
- Page 281 and 282:
PP-II-28 USING OF
- Page 283 and 284:
AKSOYLU Ahmet Erhan Department of C
- Page 285 and 286:
DIDENKO Olga Pisarzhevskii Institut
- Page 287 and 288:
KOGAN Victor M. Zelinsky Institute
- Page 289 and 290:
NOUGMANOV Evgeniy Lomonosov Moscow
- Page 291 and 292:
SERP Philippe INPToulouse 118 Route
- Page 293 and 294:
ZAITSEV Yurij P. Institute for Sorp
- Page 295 and 296:
steel, Hastelloy C22, Titanium, Tan
- Page 297:
Условия эксплуатац
- Page 305 and 306:
DONAU LAB MOSCOW ПРОГРАММА
- Page 307 and 308:
CONTENT PLENARY LECTURES...........
- Page 309 and 310:
OP-I-12 Maldonado-Hódar F.J., Carr
- Page 311 and 312:
OP-II-8 Isse A.A.,
- Page 313 and 314:
PP-I-21 Karaseva M.S., Perederiy M.
- Page 315 and 316:
PP-I-47 Zaitsev Yu., Zhuravsky S.,
- Page 317 and 318:
PP-II-21 Özkara S