II International Symposium on Carbon for Catalysis ABSTRACTS
II International Symposium on Carbon for Catalysis ABSTRACTS
II International Symposium on Carbon for Catalysis ABSTRACTS
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
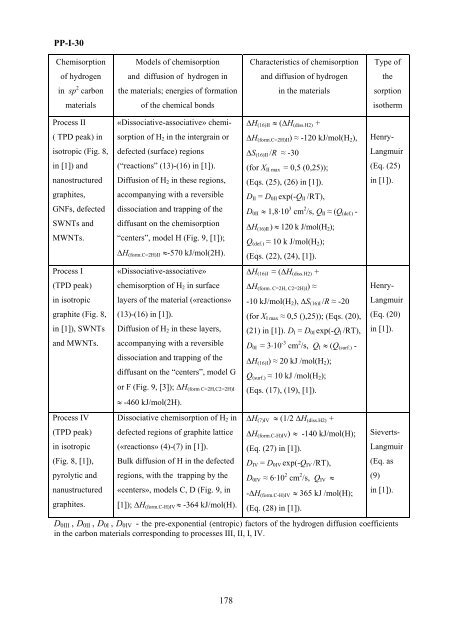
PP-I-30<br />
Chemisorpti<strong>on</strong><br />
Models of chemisorpti<strong>on</strong><br />
Characteristics of chemisorpti<strong>on</strong><br />
Type of<br />
of hydrogen<br />
and diffusi<strong>on</strong> of hydrogen in<br />
and diffusi<strong>on</strong> of hydrogen<br />
the<br />
in sp 2 carb<strong>on</strong><br />
the materials; energies of <strong>for</strong>mati<strong>on</strong><br />
in the materials<br />
sorpti<strong>on</strong><br />
materials<br />
of the chemical b<strong>on</strong>ds<br />
isotherm<br />
Process <str<strong>on</strong>g>II</str<strong>on</strong>g><br />
( TPD peak) in<br />
isotropic (Fig. 8,<br />
in [1]) and<br />
nanostructured<br />
graphites,<br />
GNFs, defected<br />
SWNTs and<br />
MWNTs.<br />
Process I<br />
(TPD peak)<br />
in isotropic<br />
graphite (Fig. 8,<br />
in [1]), SWNTs<br />
and MWNTs.<br />
Process IV<br />
(TPD peak)<br />
in isotropic<br />
(Fig. 8, [1]),<br />
pyrolytic and<br />
nanustructured<br />
graphites.<br />
«Dissociative-associative» chemisorpti<strong>on</strong><br />
of H 2 in the intergrain or<br />
defected (surface) regi<strong>on</strong>s<br />
(“reacti<strong>on</strong>s” (13)-(16) in [1]).<br />
Diffusi<strong>on</strong> of H 2 in these regi<strong>on</strong>s,<br />
accompanying with a reversible<br />
dissociati<strong>on</strong> and trapping of the<br />
diffusant <strong>on</strong> the chemisorpti<strong>on</strong><br />
“centers”, model H (Fig. 9, [1]);<br />
ΔH (<strong>for</strong>m.C=2H)<str<strong>on</strong>g>II</str<strong>on</strong>g> ≈-570 kJ/mol(2H).<br />
«Dissociative-associative»<br />
chemisorpti<strong>on</strong> of H 2 in surface<br />
layers of the material («reacti<strong>on</strong>s»<br />
(13)-(16) in [1]).<br />
Diffusi<strong>on</strong> of H 2 in these layers,<br />
accompanying with a reversible<br />
dissociati<strong>on</strong> and trapping of the<br />
diffusant <strong>on</strong> the “centers”, model G<br />
or F (Fig. 9, [3]); ΔH (<strong>for</strong>m C=2H,C2=2H)I<br />
≈ -460 kJ/mol(2H).<br />
Dissociative chemisorpti<strong>on</strong> of H 2 in<br />
defected regi<strong>on</strong>s of graphite lattice<br />
(«reacti<strong>on</strong>s» (4)-(7) in [1]).<br />
Bulk diffusi<strong>on</strong> of H in the defected<br />
regi<strong>on</strong>s, with the trapping by the<br />
«centers», models C, D (Fig. 9, in<br />
[1]); ΔH (<strong>for</strong>m.C-H)IV ≈ -364 kJ/mol(H).<br />
ΔH (16)<str<strong>on</strong>g>II</str<strong>on</strong>g> ≈ (∆H (diss.H2) +<br />
∆H (<strong>for</strong>m.C=2H)<str<strong>on</strong>g>II</str<strong>on</strong>g> ) ≈ -120 kJ/mol(H 2 ),<br />
ΔS (16)<str<strong>on</strong>g>II</str<strong>on</strong>g> /R ≈ -30<br />
(<strong>for</strong> X <str<strong>on</strong>g>II</str<strong>on</strong>g> max = 0,5 (0,25));<br />
(Eqs. (25), (26) in [1]).<br />
D <str<strong>on</strong>g>II</str<strong>on</strong>g> = D 0<str<strong>on</strong>g>II</str<strong>on</strong>g> exp(-Q <str<strong>on</strong>g>II</str<strong>on</strong>g> /RT),<br />
D 0<str<strong>on</strong>g>II</str<strong>on</strong>g> ≈ 1,8·10 3 cm 2 /s, Q <str<strong>on</strong>g>II</str<strong>on</strong>g> ≈ (Q (def.) -<br />
ΔH (16)<str<strong>on</strong>g>II</str<strong>on</strong>g> ) ≈ 120 k J/mol(H 2 );<br />
Q (def.) ≈ 10 k J/mol(H 2 );<br />
(Eqs. (22), (24), [1]).<br />
ΔH (16)I ≈ (∆H (diss.H2) +<br />
∆H (<strong>for</strong>m. C=2H, C2=2H)I ) ≈<br />
-10 kJ/mol(H 2 ), ΔS (16)I /R ≈ -20<br />
(<strong>for</strong> X I max ≈ 0,5 (),25)); (Eqs. (20),<br />
(21) in [1]). D I = D 0I exp(-Q I /RT),<br />
D 0I ≈ 3⋅10 -3 cm 2 /s, Q I ≈ (Q (surf.) -<br />
ΔH (16)I ) ≈ 20 kJ /mol(H 2 );<br />
Q (surf.) ≈ 10 kJ /mol(H 2 );<br />
(Eqs. (17), (19), [1]).<br />
ΔH (7)IV ≈ (1/2 ΔH (diss.H2) +<br />
ΔH (<strong>for</strong>m.C-H)IV ) ≈ -140 kJ/mol(H);<br />
(Eq. (27) in [1]).<br />
D IV = D 0IV exp(-Q IV /RT),<br />
D 0IV ≈ 6·10 2 cm 2 /s, Q IV ≈<br />
-ΔH (<strong>for</strong>m.C-H)IV ≈ 365 kJ /mol(H);<br />
(Eq. (28) in [1]).<br />
Henry-<br />
Langmuir<br />
(Eq. (25)<br />
in [1]).<br />
Henry-<br />
Langmuir<br />
(Eq. (20)<br />
in [1]).<br />
Sieverts-<br />
Langmuir<br />
(Eq. as<br />
(9)<br />
in [1]).<br />
D 0<str<strong>on</strong>g>II</str<strong>on</strong>g>I , D 0<str<strong>on</strong>g>II</str<strong>on</strong>g> , D 0I , D 0IV - the pre-exp<strong>on</strong>ential (entropic) factors of the hydrogen diffusi<strong>on</strong> coefficients<br />
in the carb<strong>on</strong> materials corresp<strong>on</strong>ding to processes <str<strong>on</strong>g>II</str<strong>on</strong>g>I, <str<strong>on</strong>g>II</str<strong>on</strong>g>, I, IV.<br />
178