II International Symposium on Carbon for Catalysis ABSTRACTS
II International Symposium on Carbon for Catalysis ABSTRACTS
II International Symposium on Carbon for Catalysis ABSTRACTS
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
PP-I-48<br />
Table 1 Characteristics of various solvents and measured relative wetting c<strong>on</strong>tact angles<br />
Solvent<br />
Surface<br />
tensi<strong>on</strong><br />
(mN/m)<br />
Density<br />
(kg/m 3 )<br />
Viscosity<br />
(mpa.s)<br />
Dielectric<br />
c<strong>on</strong>stant(ε0)<br />
RWCA/°<br />
Water 72.8 998.2 1.005 80 81.6<br />
Ethannol 22.8 789 1.15 24.3 24.8<br />
Acet<strong>on</strong>e 23.7 792 0.32 20.7 45.2<br />
Cyclohexane 25.3 778.6 0.97 2.016 0<br />
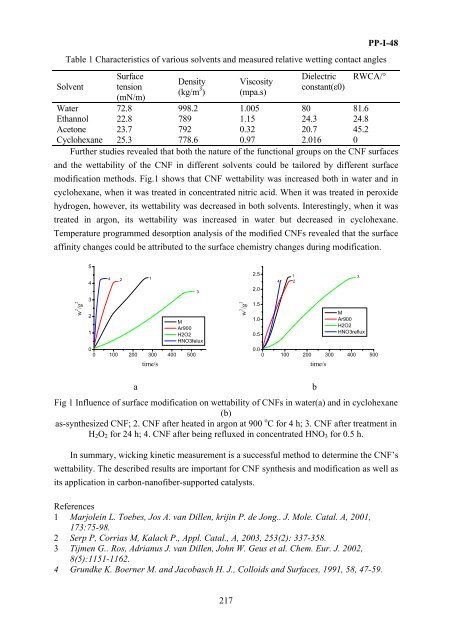
Further studies revealed that both the nature of the functi<strong>on</strong>al groups <strong>on</strong> the CNF surfaces<br />
and the wettability of the CNF in different solvents could be tailored by different surface<br />
modificati<strong>on</strong> methods. Fig.1 shows that CNF wettability was increased both in water and in<br />
cyclohexane, when it was treated in c<strong>on</strong>centrated nitric acid. When it was treated in peroxide<br />
hydrogen, however, its wettability was decreased in both solvents. Interestingly, when it was<br />
treated in arg<strong>on</strong>, its wettability was increased in water but decreased in cyclohexane.<br />
Temperature programmed desorpti<strong>on</strong> analysis of the modified CNFs revealed that the surface<br />
affinity changes could be attributed to the surface chemistry changes during modificati<strong>on</strong>.<br />
w 2 /g 2<br />
5<br />
4<br />
3<br />
4<br />
2<br />
2<br />
M<br />
Ar900<br />
1<br />
H2O2<br />
HNO3felux<br />
0<br />
0 100 200 300 400 500<br />
1<br />
time/s<br />
3<br />
w 2 /g 2<br />
2.5<br />
2.0<br />
1.5<br />
1.0<br />
0.5<br />
4<br />
1<br />
2<br />
0.0<br />
0 100 200 300 400 500<br />
time/s<br />
3<br />
M<br />
Ar900<br />
H2O2<br />
HNO3reflux<br />
a<br />
Fig 1 Influence of surface modificati<strong>on</strong> <strong>on</strong> wettability of CNFs in water(a) and in cyclohexane<br />
(b)<br />
as-synthesized CNF; 2. CNF after heated in arg<strong>on</strong> at 900 o C <strong>for</strong> 4 h; 3. CNF after treatment in<br />
H 2 O 2 <strong>for</strong> 24 h; 4. CNF after being refluxed in c<strong>on</strong>centrated HNO 3 <strong>for</strong> 0.5 h.<br />
In summary, wicking kinetic measurement is a successful method to determine the CNF’s<br />
wettability. The described results are important <strong>for</strong> CNF synthesis and modificati<strong>on</strong> as well as<br />
its applicati<strong>on</strong> in carb<strong>on</strong>-nanofiber-supported catalysts.<br />
References<br />
1 Marjolein L. Toebes, Jos A. van Dillen, krijin P. de J<strong>on</strong>g.. J. Mole. Catal. A, 2001,<br />
173:75-98.<br />
2 Serp P, Corrias M, Kalack P., Appl. Catal., A, 2003, 253(2): 337-358.<br />
3 Tijmen G.. Ros, Adrianus J. van Dillen, John W. Geus et al. Chem. Eur. J. 2002,<br />
8(5):1151-1162.<br />
4 Grundke K. Boerner M. and Jacobasch H. J., Colloids and Surfaces, 1991, 58, 47-59.<br />
b<br />
217