N-(1,3-Dimethylbutyl)-N
N-(1,3-Dimethylbutyl)-N
N-(1,3-Dimethylbutyl)-N
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
OECD SIDS<br />
N-(1,3-DIMETHYLBUTYL)-N´-PHENYL-1,4-PHENYLENEDIAMINE<br />
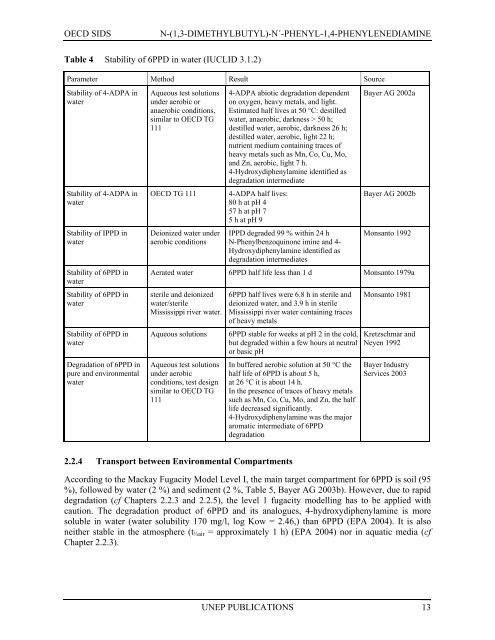
Table 4 Stability of 6PPD in water (IUCLID 3.1.2)<br />
Parameter Method Result Source<br />
Stability of 4-ADPA in<br />
water<br />
Stability of 4-ADPA in<br />
water<br />
Stability of IPPD in<br />
water<br />
Stability of 6PPD in<br />
water<br />
Stability of 6PPD in<br />
water<br />
Stability of 6PPD in<br />
water<br />
Degradation of 6PPD in<br />
pure and environmental<br />
water<br />
Aqueous test solutions<br />
under aerobic or<br />
anaerobic conditions,<br />
similar to OECD TG<br />
111<br />
OECD TG 111<br />
Deionized water under<br />
aerobic conditions<br />
4-ADPA abiotic degradation dependent<br />
on oxygen, heavy metals, and light.<br />
Estimated half lives at 50 °C: destilled<br />
water, anaerobic, darkness > 50 h;<br />
destilled water, aerobic, darkness 26 h;<br />
destilled water, aerobic, light 22 h;<br />
nutrient medium containing traces of<br />
heavy metals such as Mn, Co, Cu, Mo,<br />
and Zn, aerobic, light 7 h.<br />
4-Hydroxydiphenylamine identified as<br />
degradation intermediate<br />
4-ADPA half lives:<br />
80 h at pH 4<br />
57 h at pH 7<br />
5 h at pH 9<br />
IPPD degraded 99 % within 24 h<br />
N-Phenylbenzoquinone imine and 4-<br />
Hydroxydiphenylamine identified as<br />
degradation intermediates<br />
Bayer AG 2002a<br />
Bayer AG 2002b<br />
Monsanto 1992<br />
Aerated water 6PPD half life less than 1 d Monsanto 1979a<br />
sterile and deionized<br />
water/sterile<br />
Mississippi river water.<br />
Aqueous solutions<br />
Aqueous test solutions<br />
under aerobic<br />
conditions, test design<br />
similar to OECD TG<br />
111<br />
6PPD half lives were 6.8 h in sterile and<br />
deionized water, and 3.9 h in sterile<br />
Mississippi river water containing traces<br />
of heavy metals<br />
6PPD stable for weeks at pH 2 in the cold,<br />
but degraded within a few hours at neutral<br />
or basic pH<br />
In buffered aerobic solution at 50 °C the<br />
half life of 6PPD is about 5 h,<br />
at 26 °C it is about 14 h.<br />
In the presence of traces of heavy metals<br />
such as Mn, Co, Cu, Mo, and Zn, the half<br />
life decreased significantly.<br />
4-Hydroxydiphenylamine was the major<br />
aromatic intermediate of 6PPD<br />
degradation<br />
Monsanto 1981<br />
Kretzschmar and<br />
Neyen 1992<br />
Bayer Industry<br />
Services 2003<br />
2.2.4 Transport between Environmental Compartments<br />
According to the Mackay Fugacity Model Level I, the main target compartment for 6PPD is soil (95<br />
%), followed by water (2 %) and sediment (2 %, Table 5, Bayer AG 2003b). However, due to rapid<br />
degradation (cf Chapters 2.2.3 and 2.2.5), the level 1 fugacity modelling has to be applied with<br />
caution. The degradation product of 6PPD and its analogues, 4-hydroxydiphenylamine is more<br />
soluble in water (water solubility 170 mg/l, log Kow = 2.46,) than 6PPD (EPA 2004). It is also<br />
neither stable in the atmosphere (t ½air = approximately 1 h) (EPA 2004) nor in aquatic media (cf<br />
Chapter 2.2.3).<br />
UNEP PUBLICATIONS 13