2013 Promega catalogue
2013 Promega catalogue
2013 Promega catalogue
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
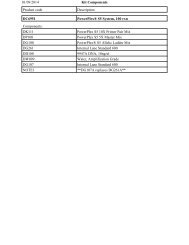
Cell Signaling<br />
DUB-Glo Protease Assay<br />
Product Size Cat.# Price ($)<br />
DUB-Glo Protease Assay (DUB/SENP/NEDP) 10 ml G6260 667<br />
For Research Use Only. Not for Use in Diagnostic Procedures.<br />
50 ml G6261 Pls. Enq.<br />
Description: The DUB-Glo Protease Assay (DUB/SENP/NEDP) is a<br />
homogeneous, bioluminescent assay that measures the activity of numerous<br />
deconjugating enzymes including deubiquitinating (DUB), deSUMOylating<br />
(SENP) and deneddylating (NEDP) proteases. These proteases reverse the<br />
protein modification by ubiquitin and ubiquitin-like proteins (Ubl proteins) and<br />
thus are integral components in the complex mechanisms of posttranslational<br />
protein regulation in eukaryotes.<br />
Features:<br />
• Greater Sensitivity: The luminescent format provides enough sensitivity<br />
to enable use of a simple peptide-based substrate, Z-RLRGG-aminoluciferin,<br />
for assaying deconjugating proteases. Fluorescence generally requires<br />
the use of full-length substrates.<br />
• Broad Dynamic Range: The assays are linear over 2–3 logs of deconjugating<br />
protease concentrations.<br />
• Signal Stability: The coupled-enzyme format results in very stable signal<br />
with a half-life >3 hours. Substrate depletion is not a concern as it is when<br />
using the full-length substrates, Ub-AMC, SUMO-AMC or Nedd8-AMC.<br />
• Fast: Maximum sensitivity is reached in 10–30 minutes after reagent<br />
addition because the signal is not dependent on accumulation of cleaved<br />
product for sensitivity in the coupled-enzyme format.<br />
• Accurate and Robust: The broad linear range and excellent sensitivity<br />
readily translate to accurate kinetic analysis of inhibitors. Assays can be<br />
scaled to 384-well with suitable Z´ factors.<br />
• Greater Flexibility: The K m values for the peptide substrates are much<br />
higher than they are for full-length substrates, yet the sensitivity of the<br />
luminescent assay allows the assay to be run significantly below K m while<br />
still achieving good signal-to-background ratios for extended time periods.<br />
A single luminescent substrate concentration can be used for a wide<br />
variety of DUB/SENP/NEDP proteases without worrying about substrate<br />
depletion or substrate inhibition.<br />
• Batch-Processing Capability: The homogeneous coupled-enzyme format<br />
results in a continuous signal, providing excellent stability and allowing<br />
plates to be read over an extended period of time.<br />
Storage Conditions: Store components at –20°C protected from light.<br />
Protocol<br />
DUB-Glo Protease Assay (DUB/SENP/NEDP) Technical Manual<br />
Part#<br />
TM319<br />
ApoTox-Glo Triplex Assay<br />
Product Size Cat.# Price ($)<br />
ApoTox-Glo Triplex Assay 10 ml G6320 828<br />
5 × 10 ml G6321 Pls. Enq.<br />
For Laboratory Use.<br />
Description: The ApoTox-Glo Triplex Assay combines three assay chemistries<br />
to easily assess viability, cytotoxicity and apoptosis events in the same<br />
cell-based assay well. First, viability and cytotoxicity are determined by measuring<br />
two differential protease biomarkers simultaneously with the addition of a<br />
single nonlytic reagent containing two peptide substrates. The live-cell protease<br />
activity is restricted to intact viable cells and is measured using a fluorogenic,<br />
cell-permeant peptide substrate (GF-AFC Substrate). The substrate enters<br />
intact cells, where it is cleaved to generate a fluorescent signal proportional<br />
to the number of living cells. This live-cell protease activity marker becomes<br />
inactive upon loss of membrane integrity and leakage into the surrounding<br />
culture medium. A second, cell-impermeant, fluorogenic peptide substrate (bis-<br />
AAF-R110 Substrate) is used simultaneously to measure dead-cell protease<br />
activity that has been released from cells that have lost membrane integrity.<br />
This results in ratiometric, inversely correlated measures of cell viability and<br />
cytotoxicity. The ratio of viable cells to dead cells is independent of cell number<br />
and, therefore, can be used to normalize data. A second reagent containing luminogenic<br />
DEVD-peptide substrate for caspase-3/7 and Ultra-Glo Recombinant<br />
Thermostable Luciferase is added. Caspase-3/7 cleavage of the substrate<br />
releases luciferin, which is a substrate for luciferase and generates light. The<br />
light output, measured with a luminometer, correlates with caspase-3/7 activation<br />
as a key indicator of apoptosis.<br />
Features:<br />
• Measure Viability, Cytotoxicity and Apoptosis in the Same Sample<br />
Well: Determine mechanism of cell death for cells in the same sample<br />
well.<br />
• Easily Implement: Assay follows a simple sequential “add-mix-measure”<br />
format.<br />
• Normalize Data with a Built-In Control: The ratio of the number of live<br />
cells/number of dead cells is independent of cell number and normalizes<br />
data. This normalization makes results more comparable well-to-well,<br />
plate-to-plate and day-to-day.<br />
• Flexible and Easily Automated: The volumes of each assay component<br />
can be scaled to meet throughput needs and is amenable to automation in<br />
96- and 384-well plates.<br />
• Improves Efficiency and Saves on Lab Budget: Reduces cell culture<br />
and labor costs by performing three assays in a single well.<br />
Storage Conditions: Store all components at –20°C protected from light.<br />
Protocol<br />
ApoTox-Glo Triplex Assay Technical Manual<br />
Part#<br />
TM322<br />
8<br />
Epigenetics<br />
Section<br />
Contents<br />
For complete and up-to-date product information visit: www.promega.com/catalog<br />
173<br />
Table of<br />
Contents