2013 Promega catalogue
2013 Promega catalogue
2013 Promega catalogue
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
I<br />
Life<br />
Science<br />
Catalog<br />
<strong>2013</strong><br />
Worldwide Contact List<br />
Cell Signaling<br />
Protein Phosphatases and<br />
Phosphatase Assays<br />
PPase-2A<br />
Product Size Cat.# Price ($)<br />
PPase-2A 25 u V6311 669<br />
For Research Use Only. Not for Use in Diagnostic Procedures.<br />
Description: Protein Phosphatase-2A (PPase-2A) is a serine/threonine phosphatase<br />
isolated from human red blood cells. It is isolated as the heterodimer<br />
of 60kDa (A) and 36kDa (C) subunits. It has the ability to dephosphorylate<br />
the α-subunit of phosphorylase kinase. With its 36–38kDa catalytic subunit,<br />
PPase-2A has broad substrate specificity and may play a regulatory role in DNA<br />
replication, transcription, protein synthesis, mitosis and glycogen metabolism.<br />
PPase-2A is stimulated in vitro by basic proteins such as protamine, histones<br />
and polylysine. The enzyme is inhibited by several environmental toxins and<br />
tumor promoters such as okadaic acid and microcystin-LR. The chemically<br />
synthesized phosphopeptide, RRA(pT)VA (available in the Ser/Thr Phosphatase<br />
Assay System, Cat.# V2460), is an excellent substrate for PPase-2A.<br />
Storage Conditions: Store at –20°C.<br />
Protocol<br />
PPase-2A Product Information<br />
PPase-2B<br />
Part#<br />
9PIV631<br />
Product Size Cat.# Price ($)<br />
PPase-2B 10 u V6361 188<br />
For Research Use Only. Not for Use in Diagnostic Procedures.<br />
Description: PPase-2B is a heterodimeric enzyme composed of a 19kDa<br />
calcium-binding subunit and a catalytic subunit (61kDa) that binds calmodulin.<br />
PPase-2B was originally identified based on its calcium- and calmodulindependent<br />
activity toward phosphorylase kinase and inhibitor-1. PPase-2B is<br />
identical to the brain protein calcineurin, which constitutes up to 1% of total<br />
brain protein. The immunosuppressive drugs FK-506 and cyclosporin A inhibit<br />
PPase-2B activity in immune cells, implicating a role for this enzyme in regulation<br />
of the immune system. PPase-2B also plays a major role in regulating<br />
secretory functions of a variety of cells.<br />
PPase-2B is less sensitive to okadaic acid than PPase-2A and PPase-1, requiring<br />
micromolar concentrations of okadaic acid for inhibition. It is not inhibited<br />
by Inhibitor-1 or Inhibitor-2. <strong>Promega</strong> PPase-2B is isolated from bovine brain.<br />
Storage Conditions: Store at –70°C.<br />
Protocol<br />
PPase-2B Product Information<br />
Part#<br />
9PIV636<br />
Non-Radioactive Phosphatase Assay<br />
Systems<br />
Product Size Cat.# Price ($)<br />
Serine/Threonine Phosphatase Assay System 96 reactions V2460 623<br />
Tyrosine Phosphatase Assay System 96 reactions V2471 623<br />
For Research Use Only. Not for Use in Diagnostic Procedures.<br />
Description: The Non-Radioactive Phosphatase Assay Systems provide a fast,<br />
convenient and flexible alternative for measuring protein phosphatase activity.<br />
These systems determine the amount of free phosphate generated in a reaction<br />
by measuring the absorbance of a molybdate:malachite green:phosphate<br />
complex. These systems allow the use of a variety of buffer conditions and<br />
substrates, including naturally phosphorylated proteins or synthetic phosphopeptides.<br />
The Serine/Threonine Phosphatase Assay System contains the<br />
chemically synthesized phosphopetide, RRA(pT)VA, a peptide substrate that<br />
is compatible with several serine/threonine phosphatases such as the Protein<br />
Phosphatases 2A, 2B, and 2C. However the supplied phosphopeptide<br />
is a poor substrate for Protein Phosphatase 1 because of its more<br />
stringent structural requirements.<br />
The Tyrosine Phosphatase Assay System contains two chemically synthesized<br />
phosphopeptides, END(pY)INASL and DADE(pY)LIPQQG, that serve as<br />
substrates for many protein tyrosine phosphatases. The effective range for the<br />
detection of phosphate released during an assay using the Phosphatase Assay<br />
Systems is 100–4,000pmol of phosphate. In addition to measuring phosphatase<br />
activity in partially fractionated and purified samples, the Phosphatase<br />
Assay Systems can also measure phosphatase activity in crude cell or tissue<br />
extracts. For this application, the high concentration of phosphate in these<br />
preparations is eliminated prior to performing the assay using the supplied Spin<br />
Columns, which rapidly and effectively remove free phosphate and other lowmolecular-weight<br />
inhibitors from the sample. In addition, a unique Molybdate<br />
Dye Additive that is combined with the Molybdate Dye Solution aids in the<br />
solubilization of proteins exposed to the acid conditions of the Molybdate Dye<br />
Solution, which alone could potentially cause precipitation of the proteins.<br />
Storage Conditions: Store the entire kit at 4°C.<br />
Protocol<br />
Serine/Threonine Phosphatase Assay System Technical Bulletin<br />
Tyrosine Phosphatase Assay System<br />
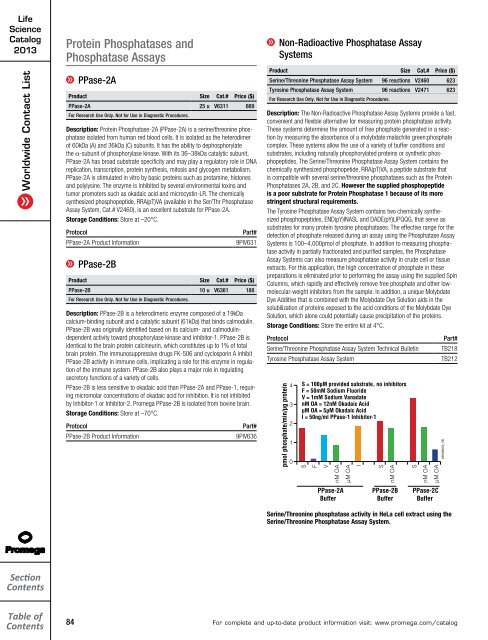
pmol phosphate/min/µg protein<br />
4<br />
3<br />
2<br />
1<br />
0<br />
S = 100µM provided substrate, no inhibitors<br />
F = 50mM Sodium Fluoride<br />
V = 1mM Sodium Vanadate<br />
nM OA = 12nM Okadaic Acid<br />
µM OA = 5µM Okadaic Acid<br />
I = 50ng/ml PPase-1 Inhibitor-1<br />
S<br />
F<br />
V<br />
nM OA<br />
µM OA<br />
S<br />
nM OA<br />
S<br />
nM OA<br />
µM OA<br />
Part#<br />
TB218<br />
TB212<br />
0960MA02_5B<br />
PPase-2A<br />
Buffer<br />
PPase-2B<br />
Buffer<br />
PPase-2C<br />
Buffer<br />
Serine/Threonine phosphatase activity in HeLa cell extract using the<br />
Serine/Threonine Phosphatase Assay System.<br />
Section<br />
Contents<br />
Table of<br />
Contents<br />
84<br />
For complete and up-to-date product information visit: www.promega.com/catalog