Phase-field modeling of diffusion controlled phase ... - KTH Mechanics
Phase-field modeling of diffusion controlled phase ... - KTH Mechanics
Phase-field modeling of diffusion controlled phase ... - KTH Mechanics
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
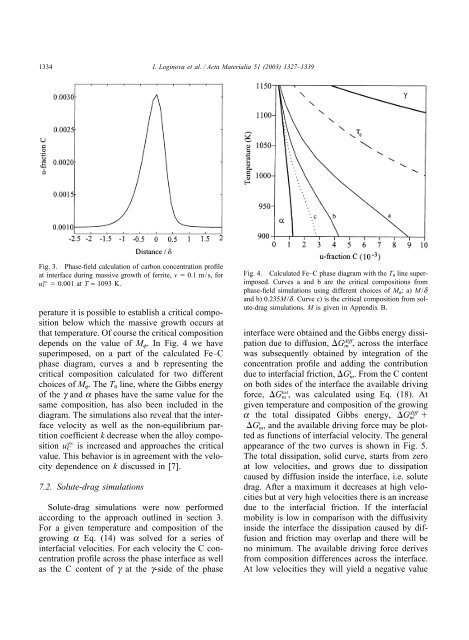
1334 I. Loginova et al. / Acta Materialia 51 (2003) 1327–1339Fig. 3. <strong>Phase</strong>-<strong>field</strong> calculation <strong>of</strong> carbon concentration pr<strong>of</strong>ileat interface during massive growth <strong>of</strong> ferrite, v 0.1 m/s, foru gC 0.001 at T = 1093 K.perature it is possible to establish a critical compositionbelow which the massive growth occurs atthat temperature. Of course the critical compositiondepends on the value <strong>of</strong> M f . In Fig. 4 we havesuperimposed, on a part <strong>of</strong> the calculated Fe–C<strong>phase</strong> diagram, curves a and b representing thecritical composition calculated for two differentchoices <strong>of</strong> M f . The T 0 line, where the Gibbs energy<strong>of</strong> the g and a <strong>phase</strong>s have the same value for thesame composition, has also been included in thediagram. The simulations also reveal that the interfacevelocity as well as the non-equilibrium partitioncoefficient k decrease when the alloy compositionu gC is increased and approaches the criticalvalue. This behavior is in agreement with the velocitydependence on k discussed in [7].7.2. Solute-drag simulationsSolute-drag simulations were now performedaccording to the approach outlined in section 3.For a given temperature and composition <strong>of</strong> thegrowing a Eq. (14) was solved for a series <strong>of</strong>interfacial velocities. For each velocity the C concentrationpr<strong>of</strong>ile across the <strong>phase</strong> interface as wellas the C content <strong>of</strong> g at the g-side <strong>of</strong> the <strong>phase</strong>Fig. 4. Calculated Fe–C <strong>phase</strong> diagram with the T 0 line superimposed.Curves a and b are the critical compositions from<strong>phase</strong>-<strong>field</strong> simulations using different choices <strong>of</strong> M f :a)M/dand b) 0.235M/d. Curve c) is the critical composition from solute-dragsimulations. M is given in Appendix B.interface were obtained and the Gibbs energy dissipationdue to <strong>diffusion</strong>, G diffm , across the interfacewas subsequently obtained by integration <strong>of</strong> theconcentration pr<strong>of</strong>ile and adding the contributiondue to interfacial friction, G i m. From the C contenton both sides <strong>of</strong> the interface the available drivingforce, G totm , was calculated using Eq. (18). Atgiven temperature and composition <strong>of</strong> the growinga the total dissipated Gibbs energy, G diffm G i m, and the available driving force may be plottedas functions <strong>of</strong> interfacial velocity. The generalappearance <strong>of</strong> the two curves is shown in Fig. 5.The total dissipation, solid curve, starts from zeroat low velocities, and grows due to dissipationcaused by <strong>diffusion</strong> inside the interface, i.e. solutedrag. After a maximum it decreases at high velocitiesbut at very high velocities there is an increasedue to the interfacial friction. If the interfacialmobility is low in comparison with the diffusivityinside the interface the dissipation caused by <strong>diffusion</strong>and friction may overlap and there will beno minimum. The available driving force derivesfrom composition differences across the interface.At low velocities they will yield a negative value