NUI Galway – UL Alliance First Annual ENGINEERING AND - ARAN ...
NUI Galway – UL Alliance First Annual ENGINEERING AND - ARAN ...
NUI Galway – UL Alliance First Annual ENGINEERING AND - ARAN ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Topographical Cues - Controlling Cellular Behavior<br />
Andrew English 1 , Niall Rooney 2 , Abhay Pandit 1 and Dimitrios Zeugolis 1<br />
1 Network of Excellence for Functional Biomaterials, National University of Ireland, <strong>Galway</strong>,<br />
Ireland<br />
2 Proxy Biomedical, <strong>Galway</strong>, Ireland, a.english1@nuigalway.ie<br />
Abstract<br />
The widespread interest in employing tissueengineered<br />
scaffolds as therapies is based on their<br />
capability to mimic native extra cellular matrix (ECM)<br />
architectures. These scaffolds should support cellular<br />
attachment, proliferation and directional growth in<br />
order to promote functional neotissue formation [1].<br />
Electrospinning has been recently introduced as a<br />
simple and versatile polymer processing method to<br />
produce sub-micron fibrous constructs with biophysical<br />
properties comparable to native ECM assemblies [2].<br />
1. Introduction<br />
Herein, we fabricated electro-spun mats with<br />
different topographies (non-aligned, aligned and<br />
porous) and evaluated the influence of topography on<br />
cell attachment, alignment and proliferation. Our data<br />
indicate that the different topographies did not affect<br />
cell viability (p>0.05), micro-machining resulted in<br />
decreased cell attachment and only aligned fibres<br />
facilitated directional cell migration.<br />
2. Materials and Methods<br />
Non-aligned and aligned electro-spun polymeric<br />
mats were fabricated as has been described previously<br />
[3]. The fibres were collected either on a static drum<br />
(non-aligned mats <strong>–</strong> Figure 1a) or on a rotating drum<br />
(aligned mats <strong>–</strong> Figure 2a). Non-aligned and aligned<br />
mats were further processed to create porosity (1c and<br />
2c respectively). Following that, all scaffolds were<br />
seeded with SAOS-2 for 14 days. Immunofluorescence<br />
images of the nuclei on the scaffold were used to<br />
quantify the effect of the topography on the scaffold.<br />
3. Results<br />
In this study, the influence of nano-topography on<br />
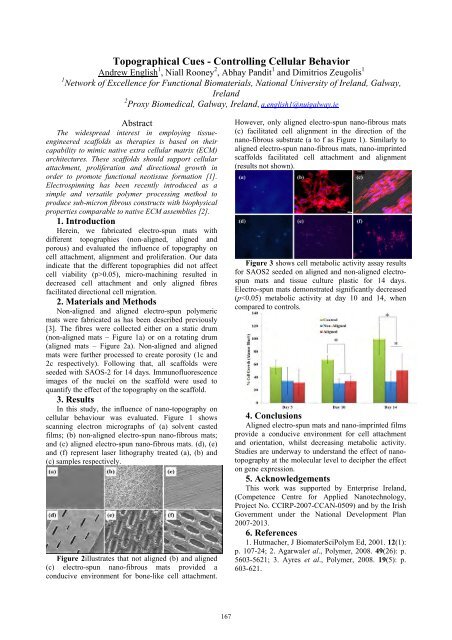
cellular behaviour was evaluated. Figure 1 shows<br />
scanning electron micrographs of (a) solvent casted<br />
films; (b) non-aligned electro-spun nano-fibrous mats;<br />
and (c) aligned electro-spun nano-fibrous mats. (d), (e)<br />
and (f) represent laser lithography treated (a), (b) and<br />
(c) samples respectively.<br />
Figure 2illustrates that not aligned (b) and aligned<br />
(c) electro-spun nano-fibrous mats provided a<br />
conducive environment for bone-like cell attachment.<br />
167<br />
However, only aligned electro-spun nano-fibrous mats<br />
(c) facilitated cell alignment in the direction of the<br />
nano-fibrous substrate (a to f as Figure 1). Similarly to<br />
aligned electro-spun nano-fibrous mats, nano-imprinted<br />
scaffolds facilitated cell attachment and alignment<br />
(results not shown).<br />
Figure 3 shows cell metabolic activity assay results<br />
for SAOS2 seeded on aligned and non-aligned electrospun<br />
mats and tissue culture plastic for 14 days.<br />
Electro-spun mats demonstrated significantly decreased<br />
(p