NUI Galway – UL Alliance First Annual ENGINEERING AND - ARAN ...
NUI Galway – UL Alliance First Annual ENGINEERING AND - ARAN ...
NUI Galway – UL Alliance First Annual ENGINEERING AND - ARAN ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Investigating the Potential of Off-the-Shelf Tissue Engineered<br />
Cardiovascular Graft Materials<br />
L.M. Davis, G.T. Carroll, A. Callanan, B.J. Doyle, M.T. Walsh, T.M. McGloughlin<br />
Centre for Applied Biomedical Engineering Research (CABER), Department of Mechanical,<br />
Aeronautical and Biomedical Engineering, Materials and Surface Science Institute (MSSI),<br />
University of Limerick, Ireland<br />
Laura.Davis@ul.ie<br />
Abstract<br />
Effective vascular tissue replacements for the treatment<br />
of cardiovascular disease are still an unaddressed<br />
worldwide problem. Naturally derived biological<br />
scaffolds offer huge potential although a major problem<br />
of using such scaffolds is storage, particularly in a<br />
hydrated and stented configuration, which can result in<br />
biomechanical changes in the scaffolds. This study<br />
analysed the mechanical and bioactive effects of<br />
hydrated storage of two scaffolds, small intestinal<br />
submucosa (SIS) and urinary bladder matrix (UBM) in<br />
both stented and un-stented configurations for up to<br />
4-months.<br />
1. Introduction<br />
Established treatment modalities for cardiovascular<br />
diseases include arterial substitutes and synthetic graft<br />
materials but these have associated issues such as low<br />
patency and compliance mismatch. Currently, there is a<br />
shift towards a tissue engineering approach in the form<br />
of acellular extracellular (ECM) based vascular grafts<br />
with such materials offering many mechanical, chemical<br />
and biological advantages over their synthetic<br />
counterparts. In order for these to be successful, a<br />
suitable biocompatible storage environment is required<br />
to allow migration, adhesion and proliferation of host<br />
cells upon material implantation.<br />
2. Methodology<br />
Multilayered ECM scaffolds (UBM and SIS) were<br />
immersed in a hydrating solution in stented and<br />
un-stented configurations simulating the catheter<br />
environment for periods of up to 4-months. Mechanical<br />
evaluation was conducted with dog-bone specimens<br />
pre-conditioned to align the material fibres and loaded<br />
until failure. Cell culture evaluation was carried out<br />
using human aortic endothelial cells (HAEC). Cellular<br />
metabolic activity and proliferation was examined using<br />
alamarBlue ® cell viability reagent for up to 96-hours in<br />
culture. Furthermore, the concentration of various<br />
nucleic acids and proteins leached during storage were<br />
analysed utilising a NanoDrop spectrophotometer.<br />
3. Results<br />
Upon deployment, uniform radial loading of the stent<br />
on the ECM samples was verified. Favourably, the<br />
average UTS of all ECM samples evaluated were noted<br />
62<br />
to be above the average aortic tissue failure strength.<br />
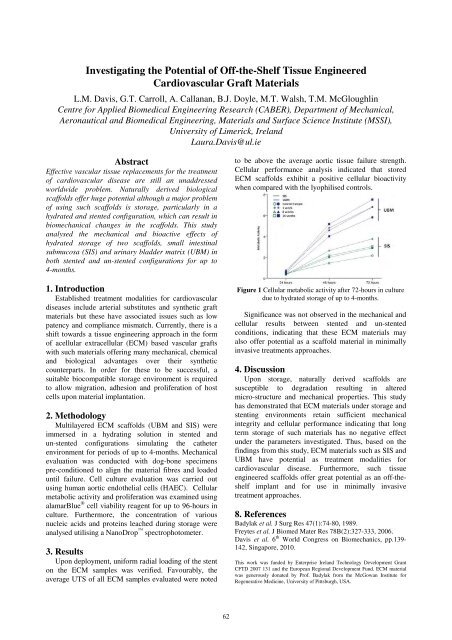
Cellular performance analysis indicated that stored<br />
ECM scaffolds exhibit a positive cellular bioactivity<br />
when compared with the lyophilised controls.<br />
Figure 1 Cellular metabolic activity after 72-hours in culture<br />
due to hydrated storage of up to 4-months.<br />
Significance was not observed in the mechanical and<br />
cellular results between stented and un-stented<br />
conditions, indicating that these ECM materials may<br />
also offer potential as a scaffold material in minimally<br />
invasive treatments approaches.<br />
4. Discussion<br />
Upon storage, naturally derived scaffolds are<br />
susceptible to degradation resulting in altered<br />
micro-structure and mechanical properties. This study<br />
has demonstrated that ECM materials under storage and<br />
stenting environments retain sufficient mechanical<br />
integrity and cellular performance indicating that long<br />
term storage of such materials has no negative effect<br />
under the parameters investigated. Thus, based on the<br />
findings from this study, ECM materials such as SIS and<br />
UBM have potential as treatment modalities for<br />
cardiovascular disease. Furthermore, such tissue<br />
engineered scaffolds offer great potential as an off-theshelf<br />
implant and for use in minimally invasive<br />
treatment approaches.<br />
8. References<br />
Badylak et al. J Surg Res 47(1):74-80, 1989.<br />
Freytes et al. J Biomed Mater Res 78B(2):327-333, 2006.<br />
Davis et al. 6 th World Congress on Biomechanics, pp.139-<br />
142, Singapore, 2010.<br />
This work was funded by Enterprise Ireland Technology Development Grant<br />
CFTD 2007 131 and the European Regional Development Fund. ECM material<br />
was generously donated by Prof. Badylak from the McGowan Institute for<br />
Regenerative Medicine, University of Pittsburgh, USA.