NUI Galway – UL Alliance First Annual ENGINEERING AND - ARAN ...
NUI Galway – UL Alliance First Annual ENGINEERING AND - ARAN ...
NUI Galway – UL Alliance First Annual ENGINEERING AND - ARAN ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Modelling Corrosion in Bioabsorbable Metallic Stents<br />
J.A. Grogan, B.J. O’ Brien, S.B. Leen, P.E. McHugh<br />
Mechanical and Biomedical Engineering and National Centre for Biomedical Engineering<br />
Science, National University of Ireland, <strong>Galway</strong>, Ireland.<br />
j.grogan1@nuigalway.ie<br />
Abstract<br />
The design of bioabsorbable stents, tiny absorbable<br />
scaffolds that are used in the treatment of heart disease,<br />
is highly challenging due to the complex mechanical<br />
and chemical interaction between them and their<br />
surroundings in the body. The aim of this work is to<br />
facilitate the development of these devices through the<br />
development and experimental calibration of a finite<br />
element based stent assessment and design tool.<br />
1. Introduction<br />
A new generation of stents that are gradually<br />
absorbed in the body are showing great promise in<br />
terms of reducing long-term health risks associated with<br />
permanent metallic implants [1]. However, the<br />
corrosion of these devices in the body is still not well<br />
understood, making their design difficult.<br />
Computational modelling is extensively used in the<br />
design of conventional, permanent stents [2]. The ability<br />
to include the effects of device corrosion in a finite<br />
element (FE) based stent design framework will greatly<br />
facilitate the development of absorbable metallic stents<br />
(AMS). This ability is developed in this work through<br />
the creation on a novel FE corrosion model and its<br />
calibration based on the results of experimental<br />
corrosion studies on a bioabsorbable alloy.<br />
2. Methods<br />
The corrosion behavior of thin biodegradable alloy<br />
(AZ31) foils is determined in simulated physiological<br />
solution, including a determination of alloy corrosion<br />
rate and the effects of mechanical loading on alloy<br />
corrosion behavior.<br />
A FE based corrosion model is developed and<br />
calibrated based on the results of the experimental<br />
corrosion tests. The model captures the effects of<br />
corrosion through the simulation of a stochastic pit<br />
growth process and the use of a corrosion damage<br />
parameter.<br />
The model is applied in simulating the corrosion of a<br />
3-D AMS geometry in a three-layer artery and is used in<br />
predicting the reduction in stent mechanical integrity<br />
over time due to corrosion.<br />
3. Results and Discussion<br />
A localized, pitting corrosion attack is observed<br />
experimentally in the alloy, resulting in a significant<br />
reduction in specimen mechanical integrity with<br />
relatively little mass loss. The effects of loading and<br />
56<br />
corrosion on foil integrity are captured very well by the<br />
FE model, as shown in Fig. 1.<br />
Fig. 1 <strong>–</strong> The experimental and simulated results of a<br />
constant load immersion test on AZ31 alloy.<br />
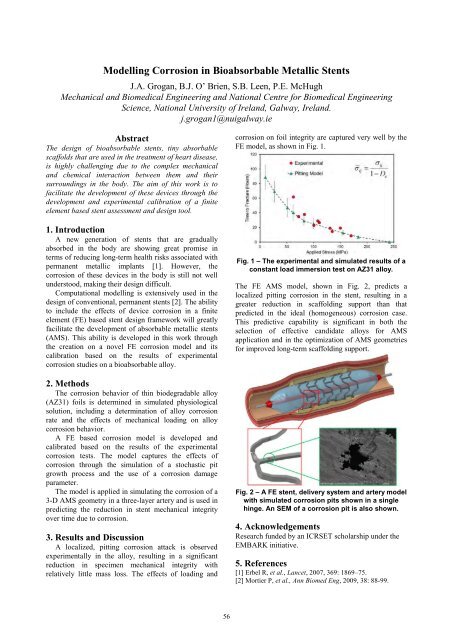
The FE AMS model, shown in Fig. 2, predicts a<br />
localized pitting corrosion in the stent, resulting in a<br />
greater reduction in scaffolding support than that<br />
predicted in the ideal (homogeneous) corrosion case.<br />
This predictive capability is significant in both the<br />
selection of effective candidate alloys for AMS<br />
application and in the optimization of AMS geometries<br />
for improved long-term scaffolding support.<br />
Fig. 2 <strong>–</strong> A FE stent, delivery system and artery model<br />
with simulated corrosion pits shown in a single<br />
hinge. An SEM of a corrosion pit is also shown.<br />
4. Acknowledgements<br />
Research funded by an ICRSET scholarship under the<br />
EMBARK initiative.<br />
5. References<br />
[1] Erbel R, et al., Lancet, 2007, 369: 1869<strong>–</strong>75.<br />
[2] Mortier P, et al., Ann Biomed Eng, 2009, 38: 88-99.