- Page 1 and 2:
4VOLUMECHEMICALTHERMODYNAMICSCHEMIC
- Page 3 and 4:
CHEMICAL THERMODYNAMICSVol. 1. Chem
- Page 5 and 6:
Published under the imprint NORTH-H
- Page 7 and 8:
viPREFACEviewed several of the othe
- Page 9 and 10:
viiiACKNOWLEDGEMENTSand Whiteshell
- Page 11 and 12:
xFOREWORDsending comments on the TD
- Page 13 and 14:
xiiCONTENTSIII Discussion of neptun
- Page 15:
xivCONTENTS11.1.4.2 Solid neptunium
- Page 18 and 19:
CONTENTSxvii20.5.1 PuSb(cr) . . . .
- Page 20 and 21:
List of Tables2.1 Symbols and termi
- Page 22 and 23:
LIST OF TABLESxxi20.2 Experimental
- Page 24 and 25:
List of Figures2.1 Standard order o
- Page 26:
Part IIntroductory material1
- Page 29 and 30:
4 1. IntroductionInorganic Complexe
- Page 31 and 32:
6 1. Introductionlected, but form a
- Page 34 and 35:
Chapter 2Standards, Conventions, an
- Page 36 and 37:
2.1 Symbols, terminology and nomenc
- Page 38 and 39:
2.1 Symbols, terminology and nomenc
- Page 40 and 41:
2.1 Symbols, terminology and nomenc
- Page 42 and 43:
2.1 Symbols, terminology and nomenc
- Page 44 and 45:
2.1 Symbols, terminology and nomenc
- Page 46 and 47:
2.1 Symbols, terminology and nomenc
- Page 48 and 49:
2.2 Units and conversion factors 23
- Page 50 and 51:
2.2 Units and conversion factors 25
- Page 52 and 53:
2.3 Standard and reference conditio
- Page 54 and 55:
2.3 Standard and reference conditio
- Page 56 and 57:
2.5 Uncertainty estimates 31Table 2
- Page 58 and 59:
2.7 Presentation of the selected da
- Page 60:
2.7 Presentation of the selected da
- Page 64 and 65:
Chapter 3Selected neptunium dataThi
- Page 66 and 67:
41Table 3.1: Selected thermodynamic
- Page 68 and 69:
43Table 3.1: (continued)Compound f
- Page 70 and 71:
45Table 3.1: (continued)Compound f
- Page 72 and 73:
47Table 3.2: Selected thermodynamic
- Page 74 and 75:
49Table 3.2: (continued)SpeciesReac
- Page 76 and 77:
51Table 3.2: (continued)SpeciesReac
- Page 78 and 79:
Chapter 4Selected plutonium dataThi
- Page 80 and 81:
55Table 4.1: Selected thermodynamic
- Page 82 and 83:
57Table 4.1: (continued)Compound f
- Page 84 and 85:
59Table 4.1: (continued)Compound f
- Page 86 and 87:
61SpeciesPu(OH) 3 (cr)ReactionTable
- Page 88 and 89:
63Table 4.2: (continued)SpeciesReac
- Page 90 and 91:
Chapter 5Selected auxiliary dataThi
- Page 92 and 93:
67Table 5.1: (continued)Compound f
- Page 94 and 95:
69Table 5.1: (continued)Compound f
- Page 96 and 97:
71Table 5.1: (continued)Compound f
- Page 98 and 99:
73Table 5.1: (continued)Compound f
- Page 100 and 101:
75Table 5.1: (continued)Compound f
- Page 102 and 103:
(footnotes continued)(p) Data from
- Page 104 and 105:
79Table 5.2: (continued)Species Rea
- Page 106:
81Table 5.2: (continued)Species Rea
- Page 110 and 111:
Chapter 6Elemental neptunium6.1 Nep
- Page 112 and 113:
6.1 Neptunium crystal and liquid 87
- Page 114:
6.2 Neptunium ideal monatomic gas 8
- Page 117 and 118:
92 7. Neptunium aqua ionsE ◦′ (
- Page 119 and 120:
94 7. Neptunium aqua ionsto the sta
- Page 121 and 122:
96 7. Neptunium aqua ions(∂ E ◦
- Page 123 and 124:
98 7. Neptunium aqua ionsnon-system
- Page 125 and 126:
100 7. Neptunium aqua ionsto the re
- Page 127 and 128:
102 7. Neptunium aqua ionsTable 7.4
- Page 130 and 131:
Chapter 8Neptunium oxygen andhydrog
- Page 132 and 133:
8.1 Aqueous neptunium hydroxide com
- Page 134 and 135:
8.1 Aqueous neptunium hydroxide com
- Page 136 and 137:
8.1 Aqueous neptunium hydroxide com
- Page 138 and 139:
8.1 Aqueous neptunium hydroxide com
- Page 140 and 141:
8.1 Aqueous neptunium hydroxide com
- Page 142 and 143:
8.2 Crystalline and amorphous neptu
- Page 144 and 145:
8.2 Crystalline and amorphous neptu
- Page 146 and 147:
8.2 Crystalline and amorphous neptu
- Page 148 and 149:
8.2 Crystalline and amorphous neptu
- Page 150 and 151:
8.2 Crystalline and amorphous neptu
- Page 152 and 153:
8.2 Crystalline and amorphous neptu
- Page 154:
8.2 Crystalline and amorphous neptu
- Page 157 and 158:
132 9. Neptunium group 17 (halogen)
- Page 159 and 160:
134 9. Neptunium group 17 (halogen)
- Page 161 and 162:
136 9. Neptunium group 17 (halogen)
- Page 163 and 164:
138 9. Neptunium group 17 (halogen)
- Page 165 and 166:
140 9. Neptunium group 17 (halogen)
- Page 167 and 168:
142 9. Neptunium group 17 (halogen)
- Page 169 and 170:
144 9. Neptunium group 17 (halogen)
- Page 171 and 172:
146 9. Neptunium group 17 (halogen)
- Page 173 and 174:
148 9. Neptunium group 17 (halogen)
- Page 175 and 176:
150 9. Neptunium group 17 (halogen)
- Page 177 and 178:
152 9. Neptunium group 17 (halogen)
- Page 179 and 180:
154 9. Neptunium group 17 (halogen)
- Page 181 and 182:
156 9. Neptunium group 17 (halogen)
- Page 183 and 184:
158 9. Neptunium group 17 (halogen)
- Page 185 and 186:
160 9. Neptunium group 17 (halogen)
- Page 187 and 188:
162 9. Neptunium group 17 (halogen)
- Page 189 and 190:
164 9. Neptunium group 17 (halogen)
- Page 191 and 192:
166 9. Neptunium group 17 (halogen)
- Page 193 and 194:
168 9. Neptunium group 17 (halogen)
- Page 195 and 196:
170 9. Neptunium group 17 (halogen)
- Page 197 and 198:
172 9. Neptunium group 17 (halogen)
- Page 199 and 200:
174 9. Neptunium group 17 (halogen)
- Page 201 and 202:
176 9. Neptunium group 17 (halogen)
- Page 203 and 204:
178 10. Neptunium group 16 compound
- Page 205 and 206:
180 10. Neptunium group 16 compound
- Page 207 and 208:
182 10. Neptunium group 16 compound
- Page 209 and 210:
184 10. Neptunium group 16 compound
- Page 212 and 213:
Chapter 11Neptunium group 15compoun
- Page 214 and 215:
11.1 Neptunium nitrogen compounds a
- Page 216 and 217:
11.1 Neptunium nitrogen compounds a
- Page 218 and 219:
11.1 Neptunium nitrogen compounds a
- Page 220 and 221:
11.1 Neptunium nitrogen compounds a
- Page 222 and 223:
11.2 Neptunium phosphorus compounds
- Page 224 and 225:
11.2 Neptunium phosphorus compounds
- Page 226 and 227:
Chapter 12Neptunium group 14compoun
- Page 228 and 229:
12.1 Neptunium carbon compounds and
- Page 230 and 231:
cal 0.3-1.6 M Na 2 SO 4 25 r H m =
- Page 232 and 233:
vlt 0 25 (0.340±0.002) (k) [95OFF/
- Page 234 and 235:
sol K + ,CO 2−3 , 20 −(2.31±0.
- Page 236 and 237:
12.1 Neptunium carbon compounds and
- Page 238 and 239:
12.1 Neptunium carbon compounds and
- Page 240 and 241:
12.1 Neptunium carbon compounds and
- Page 242 and 243:
12.1 Neptunium carbon compounds and
- Page 244 and 245:
12.1 Neptunium carbon compounds and
- Page 246 and 247:
12.1 Neptunium carbon compounds and
- Page 248 and 249:
12.1 Neptunium carbon compounds and
- Page 250 and 251:
12.1 Neptunium carbon compounds and
- Page 252 and 253:
sp 0.1 M NaCl 23 (4.68±0.03) [96RU
- Page 254 and 255:
dis 0.2 M NaClO 25 2.93 (3.42±1.0)
- Page 256 and 257:
sol 1 M NaCl 23 (2.06±0.13) [96RUN
- Page 258 and 259:
sol 0.15 M Na 2 CO 3 −(2.58±0.64
- Page 260 and 261:
sol 1 M NaClO 30 −(4.0±0.2) [93L
- Page 262 and 263:
sol 3 M NaClO 25 −(2.14±0.18)
- Page 264 and 265:
(f) Values selected in this review
- Page 266 and 267:
12.1 Neptunium carbon compounds and
- Page 268 and 269:
12.1 Neptunium carbon compounds and
- Page 270 and 271:
12.1 Neptunium carbon compounds and
- Page 272 and 273:
12.1 Neptunium carbon compounds and
- Page 274 and 275:
12.1 Neptunium carbon compounds and
- Page 276 and 277:
12.1 Neptunium carbon compounds and
- Page 278 and 279:
12.1 Neptunium carbon compounds and
- Page 280 and 281:
12.1 Neptunium carbon compounds and
- Page 282 and 283:
(V vs. SHE) (V vs. SHE) (V · K −
- Page 284 and 285:
(h) 1MK 2 CO 3 is written in Table
- Page 286 and 287:
12.1 Neptunium carbon compounds and
- Page 288 and 289:
12.1 Neptunium carbon compounds and
- Page 290 and 291:
12.1 Neptunium carbon compounds and
- Page 292 and 293:
2 3 r H m (5.0±2.0)log 10 β 2 MO
- Page 294 and 295:
12.1 Neptunium carbon compounds and
- Page 296 and 297:
12.1 Neptunium carbon compounds and
- Page 298 and 299:
12.1 Neptunium carbon compounds and
- Page 300 and 301:
12.1 Neptunium carbon compounds and
- Page 302 and 303:
12.1 Neptunium carbon compounds and
- Page 304 and 305:
12.1 Neptunium carbon compounds and
- Page 306 and 307:
12.1 Neptunium carbon compounds and
- Page 308 and 309:
12.1 Neptunium carbon compounds and
- Page 310 and 311:
12.1 Neptunium carbon compounds and
- Page 312:
12.1 Neptunium carbon compounds and
- Page 315 and 316:
290 13. Neptunium group 2 (alkaline
- Page 317 and 318:
292 14. Neptunium group 1 (alkali)
- Page 320:
Part IVDiscussion of plutonium data
- Page 323 and 324:
298 15. Elemental plutoniumTable 15
- Page 325 and 326:
300 15. Elemental plutoniumfrom 298
- Page 327 and 328:
302 16. Plutonium aqua ionsthe prop
- Page 329 and 330:
304 16. Plutonium aqua ions298.15 K
- Page 331 and 332:
306 16. Plutonium aqua ionsE ◦ =
- Page 333 and 334:
308 16. Plutonium aqua ionsSm ◦ (
- Page 335 and 336:
310 16. Plutonium aqua ionsstandard
- Page 337 and 338:
312 16. Plutonium aqua ions r H ◦
- Page 339 and 340:
314 16. Plutonium aqua ionsE ◦′
- Page 341 and 342:
316 16. Plutonium aqua ionsFigure 1
- Page 343 and 344:
318 17. Plutonium oxygen and hydrog
- Page 345 and 346:
320 17. Plutonium oxygen and hydrog
- Page 347 and 348:
322 17. Plutonium oxygen and hydrog
- Page 349 and 350:
324 17. Plutonium oxygen and hydrog
- Page 351 and 352:
1:1 pot RT 1 M NaClO 4 −5.71 [49K
- Page 353 and 354:
328 17. Plutonium oxygen and hydrog
- Page 355 and 356:
330 17. Plutonium oxygen and hydrog
- Page 357 and 358:
332 17. Plutonium oxygen and hydrog
- Page 359 and 360:
334 17. Plutonium oxygen and hydrog
- Page 361 and 362:
336 17. Plutonium oxygen and hydrog
- Page 363 and 364:
338 17. Plutonium oxygen and hydrog
- Page 365 and 366:
340 17. Plutonium oxygen and hydrog
- Page 367 and 368:
342 17. Plutonium oxygen and hydrog
- Page 370 and 371:
Chapter 18Plutonium group 17 (halog
- Page 372 and 373:
18.1 Plutonium halide compounds 347
- Page 374 and 375:
18.1 Plutonium halide compounds 349
- Page 376 and 377:
18.1 Plutonium halide compounds 351
- Page 378 and 379:
18.1 Plutonium halide compounds 353
- Page 380 and 381:
18.1 Plutonium halide compounds 355
- Page 382 and 383:
18.1 Plutonium halide compounds 357
- Page 384 and 385:
18.1 Plutonium halide compounds 359
- Page 386 and 387:
18.1 Plutonium halide compounds 361
- Page 388 and 389:
18.1 Plutonium halide compounds 363
- Page 390 and 391:
18.1 Plutonium halide compounds 365
- Page 392 and 393:
18.1 Plutonium halide compounds 367
- Page 394 and 395:
18.1 Plutonium halide compounds 369
- Page 396 and 397:
18.2 Aqueous plutonium group 17 (ha
- Page 398 and 399:
18.2 Aqueous plutonium group 17 (ha
- Page 400 and 401:
18.2 Aqueous plutonium group 17 (ha
- Page 402 and 403:
PuO 2+2+ 3F − Å PuO 2 F − (b)3
- Page 404 and 405:
18.2 Aqueous plutonium group 17 (ha
- Page 406 and 407:
18.2 Aqueous plutonium group 17 (ha
- Page 408 and 409:
18.2 Aqueous plutonium group 17 (ha
- Page 410 and 411:
18.2 Aqueous plutonium group 17 (ha
- Page 412:
18.2 Aqueous plutonium group 17 (ha
- Page 415 and 416:
390 19. Plutonium group 16 compound
- Page 417 and 418:
392 19. Plutonium group 16 compound
- Page 419 and 420:
394 19. Plutonium group 16 compound
- Page 421 and 422:
396 19. Plutonium group 16 compound
- Page 423 and 424:
398 19. Plutonium group 16 compound
- Page 426 and 427:
Chapter 20Plutonium group 15 compou
- Page 428 and 429:
20.1 Plutonium nitrogen compounds a
- Page 430 and 431:
20.1 Plutonium nitrogen compounds a
- Page 432 and 433:
Pu + NO 3Å PuNO 3sp 2 M HClO 4 20
- Page 434 and 435:
3 3 3dis, TTA 6 M (? HCl or HClO 4
- Page 436 and 437:
20.1 Plutonium nitrogen compounds a
- Page 438 and 439:
20.1 Plutonium nitrogen compounds a
- Page 440 and 441:
20.2 Plutonium phosphorus compounds
- Page 442 and 443:
20.2 Plutonium phosphorus compounds
- Page 444 and 445:
20.3 Solid plutonium phosphorus com
- Page 446 and 447:
20.4 Plutonium arsenic compounds 42
- Page 448 and 449:
20.6 The plutonium-bismuth system 4
- Page 450:
20.6 The plutonium-bismuth system 4
- Page 453 and 454:
428 21. Plutonium group 14 compound
- Page 455 and 456:
430 21. Plutonium group 14 compound
- Page 457 and 458:
432 21. Plutonium group 14 compound
- Page 459 and 460:
434 21. Plutonium group 14 compound
- Page 461 and 462:
436 21. Plutonium group 14 compound
- Page 463 and 464:
438 21. Plutonium group 14 compound
- Page 465 and 466:
440 21. Plutonium group 14 compound
- Page 467 and 468:
442 21. Plutonium group 14 compound
- Page 469 and 470:
444 21. Plutonium group 14 compound
- Page 471 and 472:
446 22. Plutonium group 2 (alkaline
- Page 474:
Chapter 23Plutonium group 1 (alkali
- Page 477 and 478:
452 BIBLIOGRAPHY[47GIN/COR][48KRA/N
- Page 479 and 480:
454 BIBLIOGRAPHY[49MAG/LAC][49ROB][
- Page 481 and 482:
456 BIBLIOGRAPHY[51WES/EYR][51WES/W
- Page 483 and 484:
458 BIBLIOGRAPHY[55COH/SUL]Cohen, D
- Page 485 and 486:
460 BIBLIOGRAPHYmetals in connectio
- Page 487 and 488:
462 BIBLIOGRAPHY[59SHE/TIM][59STU][
- Page 489 and 490:
464 BIBLIOGRAPHY[61SCH][61STO/FRY][
- Page 491 and 492:
466 BIBLIOGRAPHY[63ROB/WAL] Roberts
- Page 493 and 494:
468 BIBLIOGRAPHY[64SIL/MAR][65CHO/K
- Page 495 and 496:
470 BIBLIOGRAPHY[66DAN/ORL][66FRO/K
- Page 497 and 498:
472 BIBLIOGRAPHY[67ERM/STA][67FRL/C
- Page 499 and 500:
474 BIBLIOGRAPHY[68KRU/SAV][68KRY/K
- Page 501 and 502:
476 BIBLIOGRAPHY[69KEN/LEA][69KRY/K
- Page 503 and 504:
478 BIBLIOGRAPHY[70HIN/COB][70KRO/G
- Page 505 and 506:
480 BIBLIOGRAPHY[71MOS][71MOS2][71M
- Page 507 and 508:
482 BIBLIOGRAPHY[72MET/GUI][72NIK/S
- Page 509 and 510:
484 BIBLIOGRAPHY[73PAT/RAM2][73PIT]
- Page 511 and 512:
486 BIBLIOGRAPHY[74SAV][74SIM/VOL][
- Page 513 and 514:
488 BIBLIOGRAPHY[75OSB/FLO][75PAT/R
- Page 515 and 516:
490 BIBLIOGRAPHY298.15 K”, J. Che
- Page 517 and 518:
492 BIBLIOGRAPHY[76VOL/VIS][77BAG/R
- Page 519 and 520:
494 BIBLIOGRAPHY[78HAL/LEE][78HUR][
- Page 521 and 522:
496 BIBLIOGRAPHYof plutonium(IV)”
- Page 523 and 524:
498 BIBLIOGRAPHYNa 0.6 NpO 2 (CO 3
- Page 525 and 526:
500 BIBLIOGRAPHY[81CHI/AKH][81CIA/F
- Page 527 and 528:
502 BIBLIOGRAPHY[81WOO][82ALC/ROB][
- Page 529 and 530:
504 BIBLIOGRAPHY[83ALL][83BES/LIN][
- Page 531 and 532:
506 BIBLIOGRAPHY[83SCH/GOR]Schmidt,
- Page 533 and 534:
508 BIBLIOGRAPHY[84LEM][84LIE/KIM][
- Page 535 and 536:
510 BIBLIOGRAPHY[85CÔM][85EIS/KIM]
- Page 537 and 538:
512 BIBLIOGRAPHY[85NEW/SUL][85RAI/R
- Page 539 and 540:
514 BIBLIOGRAPHY[86FAH][86FRE/KEL]F
- Page 541 and 542:
516 BIBLIOGRAPHY[87BEN][87BEN/HOF][
- Page 543 and 544:
518 BIBLIOGRAPHY[88HIR/LIE][88ITU][
- Page 545 and 546:
520 BIBLIOGRAPHYGeochim. Cosmochim.
- Page 547 and 548:
522 BIBLIOGRAPHY[90BEN][90CAP/VIT][
- Page 549 and 550:
524 BIBLIOGRAPHY254, 258, 258, 264,
- Page 551 and 552:
526 BIBLIOGRAPHY[91MEI][91NIT][91OK
- Page 553 and 554:
528 BIBLIOGRAPHYment”, Waste Mana
- Page 555 and 556:
530 BIBLIOGRAPHY[93ERI/NDA][93GIF/V
- Page 557 and 558:
532 BIBLIOGRAPHY[93TAY/LEM][93WEG/O
- Page 559 and 560:
534 BIBLIOGRAPHYpages 222, 223, 226
- Page 561 and 562:
536 BIBLIOGRAPHYK, gas-phase infrar
- Page 563 and 564:
538 BIBLIOGRAPHY[96ALL/VEI][96CAP/V
- Page 565 and 566:
540 BIBLIOGRAPHY[97CAP/VIT][97FEL/R
- Page 567 and 568:
542 BIBLIOGRAPHY[99RAI/HES2][99RAR/
- Page 569 and 570:
544 LIST OF CITED AUTHORSTable 23.1
- Page 571 and 572:
546 LIST OF CITED AUTHORSTable 23.1
- Page 573 and 574:
548 LIST OF CITED AUTHORSTable 23.1
- Page 575 and 576:
550 LIST OF CITED AUTHORSTable 23.1
- Page 577 and 578:
552 LIST OF CITED AUTHORSTable 23.1
- Page 579 and 580:
554 LIST OF CITED AUTHORSTable 23.1
- Page 581 and 582:
556 LIST OF CITED AUTHORSTable 23.1
- Page 583 and 584:
558 LIST OF CITED AUTHORSTable 23.1
- Page 585 and 586:
560 LIST OF CITED AUTHORSTable 23.1
- Page 587 and 588:
562 LIST OF CITED AUTHORSTable 23.1
- Page 589 and 590:
564 LIST OF CITED AUTHORSTable 23.1
- Page 591 and 592:
566 LIST OF CITED AUTHORSTable 23.1
- Page 593 and 594:
568 LIST OF CITED AUTHORSTable 23.1
- Page 596 and 597:
Appendix ADiscussion of selected re
- Page 598 and 599:
A. Discussion of selected reference
- Page 600 and 601:
A. Discussion of selected reference
- Page 602 and 603:
A. Discussion of selected reference
- Page 604 and 605:
A. Discussion of selected reference
- Page 606 and 607:
A. Discussion of selected reference
- Page 608 and 609:
A. Discussion of selected reference
- Page 610 and 611:
A. Discussion of selected reference
- Page 612 and 613:
A. Discussion of selected reference
- Page 614 and 615:
A. Discussion of selected reference
- Page 616 and 617:
A. Discussion of selected reference
- Page 618 and 619:
A. Discussion of selected reference
- Page 620 and 621:
A. Discussion of selected reference
- Page 622 and 623:
A. Discussion of selected reference
- Page 624 and 625:
A. Discussion of selected reference
- Page 626 and 627:
A. Discussion of selected reference
- Page 628 and 629:
A. Discussion of selected reference
- Page 630 and 631:
A. Discussion of selected reference
- Page 632 and 633:
A. Discussion of selected reference
- Page 634 and 635:
A. Discussion of selected reference
- Page 636 and 637:
A. Discussion of selected reference
- Page 638 and 639:
A. Discussion of selected reference
- Page 640 and 641:
A. Discussion of selected reference
- Page 642 and 643:
A. Discussion of selected reference
- Page 644 and 645:
A. Discussion of selected reference
- Page 646 and 647:
A. Discussion of selected reference
- Page 648 and 649:
A. Discussion of selected reference
- Page 650 and 651:
A. Discussion of selected reference
- Page 652 and 653:
A. Discussion of selected reference
- Page 654 and 655:
A. Discussion of selected reference
- Page 656 and 657:
A. Discussion of selected reference
- Page 658 and 659:
A. Discussion of selected reference
- Page 660 and 661:
A. Discussion of selected reference
- Page 662 and 663:
A. Discussion of selected reference
- Page 664 and 665:
A. Discussion of selected reference
- Page 666 and 667:
A. Discussion of selected reference
- Page 668 and 669:
A. Discussion of selected reference
- Page 670 and 671:
A. Discussion of selected reference
- Page 672 and 673:
A. Discussion of selected reference
- Page 674 and 675:
A. Discussion of selected reference
- Page 676 and 677:
A. Discussion of selected reference
- Page 678 and 679:
A. Discussion of selected reference
- Page 680 and 681:
A. Discussion of selected reference
- Page 682 and 683:
A. Discussion of selected reference
- Page 684 and 685:
A. Discussion of selected reference
- Page 686 and 687:
A. Discussion of selected reference
- Page 688 and 689:
A. Discussion of selected reference
- Page 690 and 691:
A. Discussion of selected reference
- Page 692 and 693:
A. Discussion of selected reference
- Page 694 and 695:
A. Discussion of selected reference
- Page 696 and 697:
A. Discussion of selected reference
- Page 698 and 699:
A. Discussion of selected reference
- Page 700 and 701:
A. Discussion of selected reference
- Page 702 and 703:
A. Discussion of selected reference
- Page 704 and 705:
A. Discussion of selected reference
- Page 706 and 707:
A. Discussion of selected reference
- Page 708 and 709:
A. Discussion of selected reference
- Page 710 and 711:
A. Discussion of selected reference
- Page 712 and 713:
A. Discussion of selected reference
- Page 714 and 715:
A. Discussion of selected reference
- Page 716 and 717:
A. Discussion of selected reference
- Page 718 and 719:
A. Discussion of selected reference
- Page 720 and 721:
A. Discussion of selected reference
- Page 722 and 723:
A. Discussion of selected reference
- Page 724 and 725:
A. Discussion of selected reference
- Page 726 and 727:
A. Discussion of selected reference
- Page 728 and 729:
A. Discussion of selected reference
- Page 730 and 731:
A. Discussion of selected reference
- Page 732 and 733:
A. Discussion of selected reference
- Page 734 and 735:
A. Discussion of selected reference
- Page 736 and 737:
A. Discussion of selected reference
- Page 738 and 739:
A. Discussion of selected reference
- Page 740 and 741:
A. Discussion of selected reference
- Page 742 and 743:
A. Discussion of selected reference
- Page 744 and 745:
A. Discussion of selected reference
- Page 746 and 747:
A. Discussion of selected reference
- Page 748 and 749: A. Discussion of selected reference
- Page 750 and 751: A. Discussion of selected reference
- Page 752 and 753: A. Discussion of selected reference
- Page 754 and 755: A. Discussion of selected reference
- Page 756 and 757: A. Discussion of selected reference
- Page 758 and 759: A. Discussion of selected reference
- Page 760 and 761: A. Discussion of selected reference
- Page 762 and 763: A. Discussion of selected reference
- Page 764 and 765: A. Discussion of selected reference
- Page 766 and 767: A. Discussion of selected reference
- Page 768 and 769: A. Discussion of selected reference
- Page 770 and 771: A. Discussion of selected reference
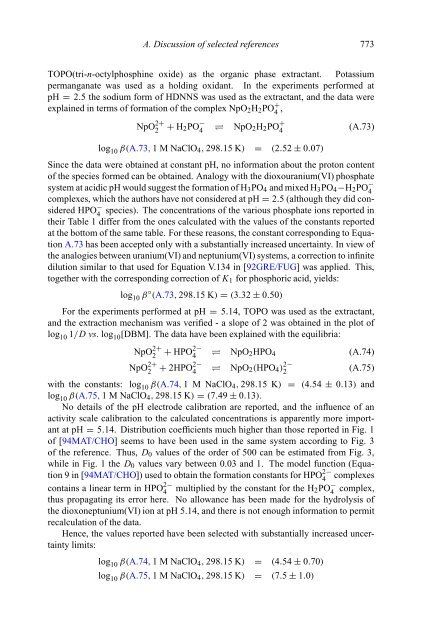
- Page 772 and 773: A. Discussion of selected reference
- Page 774 and 775: A. Discussion of selected reference
- Page 776 and 777: A. Discussion of selected reference
- Page 778 and 779: 5 −10.63±0.11 (g) −12.48±0.23
- Page 780 and 781: A. Discussion of selected reference
- Page 782 and 783: A. Discussion of selected reference
- Page 784 and 785: A. Discussion of selected reference
- Page 786 and 787: A. Discussion of selected reference
- Page 788 and 789: dataarein1MNa + media, except as no
- Page 790 and 791: A. Discussion of selected reference
- Page 792 and 793: A. Discussion of selected reference
- Page 794 and 795: A. Discussion of selected reference
- Page 796 and 797: A. Discussion of selected reference
- Page 800 and 801: A. Discussion of selected reference
- Page 802 and 803: A. Discussion of selected reference
- Page 804 and 805: A. Discussion of selected reference
- Page 806 and 807: A. Discussion of selected reference
- Page 808 and 809: A. Discussion of selected reference
- Page 810 and 811: A. Discussion of selected reference
- Page 812 and 813: A. Discussion of selected reference
- Page 814 and 815: A. Discussion of selected reference
- Page 816 and 817: A. Discussion of selected reference
- Page 818 and 819: A. Discussion of selected reference
- Page 820 and 821: A. Discussion of selected reference
- Page 822 and 823: A. Discussion of selected reference
- Page 824 and 825: Appendix BIonic strength correction
- Page 826 and 827: B.1 The specific ion interaction eq
- Page 828 and 829: B.1 The specific ion interaction eq
- Page 830 and 831: B.1 The specific ion interaction eq
- Page 832 and 833: B.1 The specific ion interaction eq
- Page 834 and 835: B.1 The specific ion interaction eq
- Page 836 and 837: B.2 Ion interaction coefficients ve
- Page 838 and 839: B.3 Tables of ion interaction coeff
- Page 840 and 841: B.3 Tables of ion interaction coeff
- Page 842 and 843: B.3 Tables of ion interaction coeff
- Page 844 and 845: B.3 Tables of ion interaction coeff
- Page 846 and 847: B.3 Tables of ion interaction coeff
- Page 848 and 849:
Appendix CAssigned uncertainties
- Page 850 and 851:
C.2 Two or more independent source
- Page 852 and 853:
C.2 Two or more independent source
- Page 854 and 855:
C.3 Several data at different ionic
- Page 856 and 857:
C.3 Several data at different ionic
- Page 858 and 859:
C.4 Procedures for data handling 83
- Page 860 and 861:
C.4 Procedures for data handling 83
- Page 862 and 863:
Index837
- Page 864 and 865:
INDEX - GENERAL 839standard state p
- Page 866 and 867:
INDEX - NEPTUNIUM 841elemental nept
- Page 868 and 869:
INDEX - PLUTONIUM 843Index - pluton
- Page 870:
INDEX - PLUTONIUM 845monobismuthide