- Page 1 and 2:
TM Food Lipids Chemistry, Nutrition
- Page 3 and 4:
FOOD SCIENCE AND TECHNOLOGY A Serie
- Page 5 and 6:
37. Omega-3 Fatty Acids in Health a
- Page 7 and 8:
90. Dairy Technology: Principles of
- Page 9 and 10:
Preface to the Second Edition Reade
- Page 11 and 12:
Preface to the First Edition There
- Page 13 and 14:
Contents Preface to the Second Edit
- Page 15 and 16:
20. Dietary Fats and Coronary Heart
- Page 17 and 18:
J. Bruce German Department of Food
- Page 19 and 20:
1 Nomenclature and Classification o
- Page 21 and 22:
described as 2-methyl-3-phytyl-1,4-
- Page 23 and 24:
Table 3 A Summary of Sequence Prior
- Page 25 and 26:
Table 5 Systematic, Common, and Sho
- Page 27 and 28:
saturation of EFA occurs (primarily
- Page 29 and 30:
Figure 5 Prostaglandin metabolites
- Page 31 and 32:
Figure 7 Prostanoic acid and prosta
- Page 33 and 34:
Figure 9 Eicosenoid isomers in part
- Page 35 and 36:
Figure 10 Nomenclature of cyclic fa
- Page 37 and 38:
Figure 13 Hydroxy fatty acid struct
- Page 39 and 40:
Figure 15 Furanoid fatty acid struc
- Page 41 and 42: Table 7 Short Abbreviations for Som
- Page 43 and 44: Figure 20 Steroid nomenclature. rep
- Page 45 and 46: Figure 22 Cholesterol oxidation pro
- Page 47 and 48: E. Phosphoglycerides (Phospholipids
- Page 49 and 50: Figure 26 Glyceroglycolipid structu
- Page 51 and 52: Figure 28 Structures of some vitami
- Page 53 and 54: Figure 29 Structures of some vitami
- Page 55 and 56: Figure 32 Structures of some vitami
- Page 57 and 58: 6. IUPAC. Nomenclature of Organic C
- Page 59 and 60: 2 Chemistry and Function of Phospho
- Page 61 and 62: variant [1]. Phosphonolipids are ma
- Page 63 and 64: nonelectrolytes, however, the perme
- Page 65 and 66: pensations in that enthalpy lost by
- Page 67 and 68: Table 3 Membrane Deterioration in A
- Page 69 and 70: erols to generate semisolid or plas
- Page 71 and 72: The interaction of phospholipids wi
- Page 73 and 74: fatty acid occurs when Fe 3� at t
- Page 75 and 76: 4. M. C. Blok, L. L. M. van Deenen,
- Page 77 and 78: 47. N. Markova, E. Sparr, L. Wadso,
- Page 79 and 80: 87. R. J. Hsieh. Contribution of li
- Page 81 and 82: 3 Lipid-Based Emulsions and Emulsif
- Page 83 and 84: may be composed of surface-active c
- Page 85 and 86: 2. Cloud Point When a surfactant so
- Page 87 and 88: HLB=7� � (hydrophilic group num
- Page 89 and 90: sion is known as the phase inversio
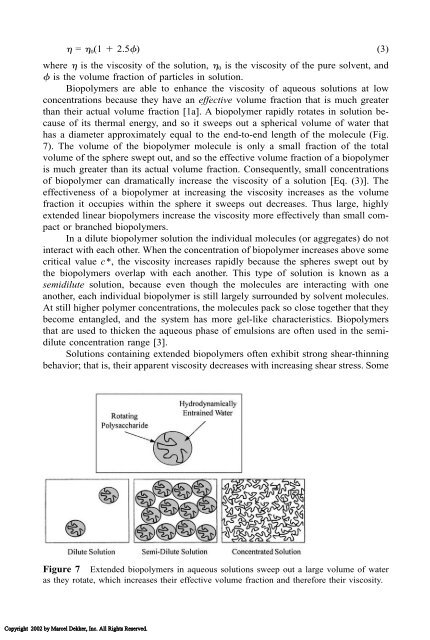
- Page 91: strongly with each other rather tha
- Page 95 and 96: Figure 8 Biopolymer molecules or ag
- Page 97 and 98: divide homogenization into two cate
- Page 99 and 100: 2� �P 1 = (4) r where � is th
- Page 101 and 102: flow rate, decreasing the size of t
- Page 103 and 104: sion be particularly small, it is u
- Page 105 and 106: 2. Electrostatic Interactions Elect
- Page 107 and 108: profile of interdroplet pair potent
- Page 109 and 110: Figure 12 Mechanisms of emulsion in
- Page 111 and 112: [Eq. (9)], but at high droplet conc
- Page 113 and 114: alteration in the system’s compos
- Page 115 and 116: Food emulsions always contain dropl
- Page 117 and 118: creaming and sedimentation in emuls
- Page 119 and 120: Foams (E. Dickinson and G. Stainsby
- Page 121 and 122: 4 The Chemistry of Waxes and Sterol
- Page 123 and 124: Shrinkage and flash point are two f
- Page 125 and 126: lowing discussion on chemical analy
- Page 127 and 128: violet chromophore. Application of
- Page 129 and 130: Figure 1 Examples of naturally occu
- Page 131 and 132: Scheme 1 Synthesis of mevalonic aci
- Page 133 and 134: Scheme 3 Synthesis of farnesyl pyro
- Page 135 and 136: Scheme 6 Biosynthesis of cholestero
- Page 137 and 138: educe the risk of coronary heart di
- Page 139 and 140: of cholesterol to bile acids (Schem
- Page 141 and 142: Scheme 10 Metabolic alterations of
- Page 143 and 144:
sample preparation is usually emplo
- Page 145 and 146:
at levels of 1-100 �g per compone
- Page 147 and 148:
30. H. W. Chen, A. A. Kandutsch, an
- Page 149 and 150:
Biologically Significant Steroids (
- Page 151 and 152:
5 Extraction and Analysis of Lipids
- Page 153 and 154:
preparing nutritional labeling mate
- Page 155 and 156:
polar solvents, such as alkanols, f
- Page 157 and 158:
a ternary system consisting of chlo
- Page 159 and 160:
lipids from meat or hydrolytic prod
- Page 161 and 162:
perature under vacuum. Acid hydroly
- Page 163 and 164:
IV. ANALYSIS OF LIPID EXTRACTS Lipi
- Page 165 and 166:
ments, etc.) primarily involves chr
- Page 167 and 168:
2. Gas Chromatography The GC (or GL
- Page 169 and 170:
4. Supercritical Fluid Chromatograp
- Page 171 and 172:
Table 3 Solvent Systems that Could
- Page 173 and 174:
sibility of determining all compone
- Page 175 and 176:
the isolated trans band is another
- Page 177 and 178:
ionized molecule has the highest m/
- Page 179 and 180:
3. W. R. Bloor. Outline of a classi
- Page 181 and 182:
45. Association of Official Analyti
- Page 183 and 184:
90. M. N. Vaghela and A. Kilara. A
- Page 185 and 186:
132. C. G. Walton, W. M. N. Ratnaya
- Page 187 and 188:
6 Methods for trans Fatty Acid Anal
- Page 189 and 190:
where A = abc (1) 1 A = absorbance
- Page 191 and 192:
methyl elaidate weight equivalents
- Page 193 and 194:
method was modified by inclusion of
- Page 195 and 196:
may be packed or bound to a column,
- Page 197 and 198:
Figure 4 The C-18 region of the gas
- Page 199 and 200:
Table 2 Response Factors of Unsatur
- Page 201 and 202:
Figure 5 Separation of the phenacyl
- Page 203 and 204:
B. Gas Chromatography/IR Spectrosco
- Page 205 and 206:
Figure 7 Expanded IR spectral range
- Page 207 and 208:
CFAMs before converting them to the
- Page 209 and 210:
Figure 8 GC-EIMS chromatographic da
- Page 211 and 212:
flame ionization detection. The fat
- Page 213 and 214:
levels in excess of 50% of the tota
- Page 215 and 216:
22. A. Huang and D. Firestone. Dete
- Page 217 and 218:
59. E. G. Perkins and C. Smick. Oct
- Page 219 and 220:
97. Association of Official Analyti
- Page 221 and 222:
136. J. J. Myer and A. Kukis. Elect
- Page 223 and 224:
7 Chemistry of Frying Oils KATHLEEN
- Page 225 and 226:
Table 1 Effects of Physical and Che
- Page 227 and 228:
Figure 3 Oxidation reactions in fry
- Page 229 and 230:
or intermittent frying, oil filtrat
- Page 231 and 232:
for the ultimate criteria to evalua
- Page 233 and 234:
triacylglycerol polymers, and triac
- Page 235 and 236:
100 compounds identified in hydroge
- Page 237 and 238:
5. J. Pokorny. Flavor chemistry of
- Page 239 and 240:
50. Anonymous. Recommendations of t
- Page 241 and 242:
8 Recovery, Refining, Converting, a
- Page 243 and 244:
Figure 1 Depiction of hard screw pr
- Page 245 and 246:
Figure 2 Depiction of prepress solv
- Page 247 and 248:
Seed containing more than the criti
- Page 249 and 250:
Figure 5 Steps in processing soybea
- Page 251 and 252:
specification of 50% protein is met
- Page 253 and 254:
tent of prepress cake is 15-18%, an
- Page 255 and 256:
in successive passes through the be
- Page 257 and 258:
Figure 10 Additional commonly used
- Page 259 and 260:
that follow the DT. Drying at norma
- Page 261 and 262:
B. Extraction of Oil-Bearing Fruits
- Page 263 and 264:
1. Wet Rendering Wet rendering is t
- Page 265 and 266:
Figure 15 Process flow sheet for de
- Page 267 and 268:
Table 2 Properties of Some Crude an
- Page 269 and 270:
Figure 16 Process flow sheet for al
- Page 271 and 272:
Figure 17 Process flow sheet for va
- Page 273 and 274:
for problematic high wax contents (
- Page 275 and 276:
Figure 19 Oil processing facilities
- Page 277 and 278:
Figure 20 Depiction of physical ref
- Page 279 and 280:
4-7�C, and then to tanks with slo
- Page 281 and 282:
Figure 21 Hydrogenation reaction me
- Page 283 and 284:
ond, which may form in its original
- Page 285 and 286:
Figure 24 Plasticization of margari
- Page 287 and 288:
Figure 25 Equipment used in expande
- Page 289 and 290:
selectivity, others claim success i
- Page 291 and 292:
48. E. J. Campbell. Sunflower oil.
- Page 293 and 294:
9 Crystallization and Polymorphism
- Page 295 and 296:
Generally, it is accepted that both
- Page 297 and 298:
Figure 2 A point lattice. (Adapted
- Page 299 and 300:
structure.... The term ‘fat’ us
- Page 301 and 302:
Figure 6 Schematic representation o
- Page 303 and 304:
Figure 8 Polymorphic transitions of
- Page 305 and 306:
where A = B = C and all are saturat
- Page 307 and 308:
LMF was found to facilitate the tra
- Page 309 and 310:
Table 3 Nomenclature and Melting Po
- Page 311 and 312:
Other mixed-fat systems have been s
- Page 313 and 314:
Figure 12 Proposed intermediate str
- Page 315 and 316:
17. K. Larsson. The crystal structu
- Page 317 and 318:
59. G. G. Jewell. Vegetable fats. I
- Page 319 and 320:
10 Chemical Interesterification of
- Page 321 and 322:
however, since monoacylglycerols an
- Page 323 and 324:
D. The ‘‘Real’’ Catalyst Th
- Page 325 and 326:
Figure 3 Proposed reaction mechanis
- Page 327 and 328:
Figure 5 Kinetics of interesterific
- Page 329 and 330:
Table 1 Theoretical Triacylglycerol
- Page 331 and 332:
Cast et al. [55] demonstrated the r
- Page 333 and 334:
Solid: Liquid: SSS, 33.3% OOO, 8.3%
- Page 335 and 336:
Figure 11 Changes in the fatty acid
- Page 337 and 338:
fication and blending on butterfat-
- Page 339 and 340:
influence of interesterification on
- Page 341 and 342:
B. Margarines In the manufacture of
- Page 343 and 344:
Figure 16 Proportion of soild fat o
- Page 345 and 346:
Tautorus and McCurdy [102] demonstr
- Page 347 and 348:
ACKNOWLEDGMENTS The authors acknowl
- Page 349 and 350:
54. A. Kuksis, M. J. McCarthy, and
- Page 351 and 352:
101. S. Zalewski and A. M. Gaddis.
- Page 353 and 354:
11 Lipid Oxidation of Edible Oil DA
- Page 355 and 356:
Figure 1 Molecular orbital of tripl
- Page 357 and 358:
Figure 3 Singlet oxygen formation b
- Page 359 and 360:
tween substrate and triplet oxygen
- Page 361 and 362:
Figure 8 Conjugated and nonconjugat
- Page 363 and 364:
Figure 11 Conjugated hydroperoxide
- Page 365 and 366:
etween the oxygen and the oxygen of
- Page 367 and 368:
cadienal, trans,trans-2,4-decadiena
- Page 369 and 370:
Figure 14 Mechanism for the formati
- Page 371 and 372:
Copyright 2002 by Marcel Dekker, In
- Page 373 and 374:
Figure 18 Formation and reactions o
- Page 375 and 376:
Figure 19 Effect of 0, 0.25, 0.5, a
- Page 377 and 378:
Figure 21 Singlet oxygen quenching
- Page 379 and 380:
10. E. N. Frankel. Chemistry of aut
- Page 381 and 382:
52. A. L. Callison. Singlet oxygen
- Page 383 and 384:
12 Lipid Oxidation of Muscle Foods
- Page 385 and 386:
A. Initiation The direct reaction o
- Page 387 and 388:
undles), and endomysia (sheaths of
- Page 389 and 390:
sosomes, etc. A comparison of the l
- Page 391 and 392:
dative stability, comparisons betwe
- Page 393 and 394:
5. Hydrolysis of Lipids and Associa
- Page 395 and 396:
of iron from the heme pocket by coo
- Page 397 and 398:
Membrane systems that reduce iron c
- Page 399 and 400:
phases of storage [51,187,188]. In
- Page 401 and 402:
4. Glutathione While the traditiona
- Page 403 and 404:
G. Mathematical Modeling The pathwa
- Page 405 and 406:
with this chemical, treated salmon
- Page 407 and 408:
multicomponent alternatives [304,30
- Page 409 and 410:
and its extent was related to inten
- Page 411 and 412:
esponded similarly to vacuum packag
- Page 413 and 414:
31. M. L. Greaser, R. G. Cassens, W
- Page 415 and 416:
72. J. S. Elmore, D. S. Mottram, M.
- Page 417 and 418:
112. J. Kanner, H. Mendel, and P. B
- Page 419 and 420:
154. M. B. Korycka-Dahl and T. Rich
- Page 421 and 422:
193. P. Akhtar, J. I. Gray, T. H. C
- Page 423 and 424:
230. B. Bjerkeng and G. Johnsen. Fr
- Page 425 and 426:
271. C.-J. Huang, and M.-L. Fwu. De
- Page 427 and 428:
313. M. G. Mast and J. H. MacNeil.
- Page 429 and 430:
353. H. A. Ghanbari, W. B. Wheeler,
- Page 431 and 432:
13 Fatty Acid Oxidation in Plant Ti
- Page 433 and 434:
1. Fatty Acid Activation Prior to d
- Page 435 and 436:
L.) leaf peroxisomes exhibits highe
- Page 437 and 438:
Figure 2 The glyoxylate cycle in gl
- Page 439 and 440:
C. �-Oxidation of Specific Fatty
- Page 441 and 442:
cotyledons and partially purified.
- Page 443 and 444:
Catabolism of heptanoyl CoA as well
- Page 445 and 446:
Figure 6 Peroxisome catabolism of m
- Page 447 and 448:
onstrated that �-oxidations requi
- Page 449 and 450:
that the member of the enzyme famil
- Page 451 and 452:
Plant oxylipin pathway, also named
- Page 453 and 454:
Figure 9 Proposed scheme for lipoxy
- Page 455 and 456:
oleic acid. These results suggest t
- Page 457 and 458:
of the seed pod reversed senescnece
- Page 459 and 460:
Figure 11 ‘‘Heterolytic’’-t
- Page 461 and 462:
Since 1971, when this physiologic r
- Page 463 and 464:
Table 1 Physiological Effects of Ja
- Page 465 and 466:
erides, although PUFAs in both form
- Page 467 and 468:
9-hydroperoxides and did not attack
- Page 469 and 470:
aerial parts of plants, constitutes
- Page 471 and 472:
32. J. B. Ohlrogge and V. S. Eccles
- Page 473 and 474:
74. L. J. Morris. The mechanism of
- Page 475 and 476:
115. B. A. Vick. Oxygenated fatty a
- Page 477 and 478:
153. T. K. Peterman and J. N. Siedo
- Page 479 and 480:
193. B. A. Stelmach, A. Müller, P.
- Page 481 and 482:
230. M. Hamberg, C. A. Herman, and
- Page 483 and 484:
14 Methods for Measuring Oxidative
- Page 485 and 486:
ated fatty acids during oxidation (
- Page 487 and 488:
Several other chemical methods have
- Page 489 and 490:
Figure 3 Relationship between perox
- Page 491 and 492:
distillate. In case of the distilla
- Page 493 and 494:
and ketones. This ion is formed fro
- Page 495 and 496:
(Fig. 9), foaming, color, viscosity
- Page 497 and 498:
Yen and Duh [69] and Chen and Ho [7
- Page 499 and 500:
Figure 10 1 H Nuclear magnetic reso
- Page 501 and 502:
to Marquez-Ruiz et al. [93], who us
- Page 503 and 504:
35. F. Shahidi, J. Yun, L.J. Rubin,
- Page 505 and 506:
77. H. Saito and K. Nakamura. Appli
- Page 507 and 508:
15 Antioxidants DAVID W. REISCHE Th
- Page 509 and 510:
Hydroperoxide degradation leads to
- Page 511 and 512:
e cyclical, with regeneration of th
- Page 513 and 514:
A. Synthetic Antioxidants Synthetic
- Page 515 and 516:
Propyl gallate (PG) 212.20 White cr
- Page 517 and 518:
4. 6-Ethoxy-1,2-dihydro-2,2,4-trime
- Page 519 and 520:
Figure 3 Structures of tocopherols
- Page 521 and 522:
Figure 4 Structures of ascorbic aci
- Page 523 and 524:
4. Enzymatic Antioxidants Glucose o
- Page 525 and 526:
would be imprudent to discount any
- Page 527 and 528:
droxycoumarin (scopoletin), and hyd
- Page 529 and 530:
Figure 7 Structures of sesame antio
- Page 531 and 532:
8. G. Minotti. Sources and role of
- Page 533 and 534:
50. K. Shimada, H. Muta, Y. Nakamur
- Page 535 and 536:
16 Antioxidant Mechanisms ERIC A. D
- Page 537 and 538:
For instance, the hydrogen of the h
- Page 539 and 540:
Figure 2 Mechanism by which one phe
- Page 541 and 542:
Figure 4 Formation of �-tocophero
- Page 543 and 544:
Figure 6 Formation of an epoxyquino
- Page 545 and 546:
gallate. The antioxidant mechanism
- Page 547 and 548:
Figure 8 Products formed from the o
- Page 549 and 550:
in food systems, transition metals
- Page 551 and 552:
An intersystem energy transfer occu
- Page 553 and 554:
V. ALTERATIONS IN LIPID OXIDATION B
- Page 555 and 556:
VII. ANTIOXIDANT INTERACTIONS Biolo
- Page 557 and 558:
26. J. Kanner, J. B. German, and J.
- Page 559 and 560:
68. J. Kanner, F. Sofer, S. Harel,
- Page 561 and 562:
17 Fats and Oils in Human Health DA
- Page 563 and 564:
Table 1 Classification of LDL Parti
- Page 565 and 566:
stearic) had been incorporated by i
- Page 567 and 568:
studies contains equal amounts (40-
- Page 569 and 570:
Table 5 Influence of 25% Caloric Re
- Page 571 and 572:
26. L. D. Cowan, D. L. O’Connell,
- Page 573 and 574:
fatty acid margarine on serum lipid
- Page 575 and 576:
nutrition examination survey. I. Ep
- Page 577 and 578:
18 Unsaturated Fatty Acids STEVEN M
- Page 579 and 580:
Figure 1 A generalized scheme for h
- Page 581 and 582:
Plant fatty acids provide a seminal
- Page 583 and 584:
plants [27]. However, the requireme
- Page 585 and 586:
The reciprocal response of the �6
- Page 587 and 588:
fatty acids and a dynamic system fo
- Page 589 and 590:
production of commercially viable o
- Page 591 and 592:
acids synthesized de novo, primaril
- Page 593 and 594:
Figure 4 The cis and trans configur
- Page 595 and 596:
VI. SYNTHESIS AND ABUNDANCE OF PUFA
- Page 597 and 598:
of eicosanoids. It is present in al
- Page 599 and 600:
usual NMIFA structures with potenti
- Page 601 and 602:
10. S. P. Baykousheva, D. L. Luthri
- Page 603 and 604:
47. M. J. T. Alaniz, I. N. T. d. Go
- Page 605 and 606:
86. R. J. Henderson and D. R. Toche
- Page 607 and 608:
19 Dietary Fats, Eicosanoids, and t
- Page 609 and 610:
synthesize EPA from linolenic acid
- Page 611 and 612:
Figure 2 Immune responses as a func
- Page 613 and 614:
done predominantly in rodent specie
- Page 615 and 616:
guinea pigs showed increased immune
- Page 617 and 618:
of energy from fat. Feeding the low
- Page 619 and 620:
14. P. Purasiri, A. Murray, S. Rich
- Page 621 and 622:
20 Dietary Fats and Coronary Heart
- Page 623 and 624:
(HDLs). Each class has its own char
- Page 625 and 626:
70% of the total amount of choleste
- Page 627 and 628:
McGandy and coworkers [8] have care
- Page 629 and 630:
Figure 4 Effects of myristic and pa
- Page 631 and 632:
3. Polyunsaturated Fatty Acids Poly
- Page 633 and 634:
Figure 8 Effects of a mixture of sa
- Page 635 and 636:
chemoattractant protein-1 (MCP-1),
- Page 637 and 638:
Figure 11 In vitro LDL oxidation. F
- Page 639 and 640:
Table 4 Fatty Acid Composition of a
- Page 641 and 642:
Figure 12 Processes involved in thr
- Page 643 and 644:
as compared with a diet rich in but
- Page 645 and 646:
from cardiovascular disease [73,74]
- Page 647 and 648:
Figure 16 Schematic representation
- Page 649 and 650:
aggregation tendency induced by som
- Page 651 and 652:
35. D. R. Janero. Malondialdehyde a
- Page 653 and 654:
68. B. J. Burrl, R. M. Dougherty, D
- Page 655 and 656:
21 Conjugated Linoleic Acids: Nutri
- Page 657 and 658:
method that works optimally in all
- Page 659 and 660:
adipose tissue contained two major
- Page 661 and 662:
providing rats with 0.5% and 1% CLA
- Page 663 and 664:
with the control group. Interesting
- Page 665 and 666:
feeding. These findings suggest tha
- Page 667 and 668:
In contrast to the antioxidative pr
- Page 669 and 670:
esponsible, at least in part, for t
- Page 671 and 672:
Yamasaki et al. studied CLA and ant
- Page 673 and 674:
17. J. K. G. Kramer, P. W. Parodi,
- Page 675 and 676:
55. C. Ip, S. F. Chin, J. A. Scimec
- Page 677 and 678:
90. J. S. Munday, K. G. Thompson, a
- Page 679 and 680:
126. J. Singh, R. Hamid, and B. S.
- Page 681 and 682:
22 Dietary Fats and Obesity DOROTHY
- Page 683 and 684:
For example, some have reported tha
- Page 685 and 686:
the fat component and the other hal
- Page 687 and 688:
shows that there is a negative corr
- Page 689 and 690:
C. Influence of Dietary Fat on Fatt
- Page 691 and 692:
Figure 1 Signal transduction cascad
- Page 693 and 694:
mals indicate that high-fat feeding
- Page 695 and 696:
processes. Numerous studies provide
- Page 697 and 698:
monier (252) suggested that in cert
- Page 699 and 700:
4. National Task Force on Obesity.
- Page 701 and 702:
46. F. Lucas, K. Ackroff, and A. Sc
- Page 703 and 704:
90. D. Mela. Sensory preference for
- Page 705 and 706:
133. D. R. Romsos and G. A. Leveill
- Page 707 and 708:
169. T. Ide, H. Kobayashi, L. Ashak
- Page 709 and 710:
207. A. B. Awad and E. A. Zepp. Alt
- Page 711 and 712:
246. Y. B. Kim, R. Nakajima, T. Mat
- Page 713 and 714:
23 Lipid-Based Synthetic Fat Substi
- Page 715 and 716:
operations, and in theory, can repl
- Page 717 and 718:
Table 3 Types of Lipid-Based Fat Su
- Page 719 and 720:
Figure 1 Structure of sucrose polye
- Page 721 and 722:
Figure 3 Synthetic scheme for olest
- Page 723 and 724:
Figure 5 Structure of sorbitol poly
- Page 725 and 726:
Figure 8 Structure of raffinose pol
- Page 727 and 728:
Figure 11 Structure of methyl galac
- Page 729 and 730:
Table 4 Some Properties of Sucrose
- Page 731 and 732:
Figure 15 Structure of trialkoxytri
- Page 733 and 734:
Figure 19 Structure of polysiloxane
- Page 735 and 736:
inversely related to the degree of
- Page 737 and 738:
Table 8 Some Nutritional Uses of No
- Page 739 and 740:
genetic assay in Chinese hamster ov
- Page 741 and 742:
IX. PERSPECTIVES With the approval
- Page 743 and 744:
30. L. Osipow, F. D. Snell, D. Marr
- Page 745 and 746:
72. K. W. Miller and P. H. Long. A
- Page 747 and 748:
24 Food Applications of Lipids FRAN
- Page 749 and 750:
Table 3 Typical Fatty Acid Composit
- Page 751 and 752:
are significant differences in oil
- Page 753 and 754:
Table 6 Production and Disappearanc
- Page 755 and 756:
and margarine should be pronounced
- Page 757 and 758:
Table 8 Approximate Fatty Acid Comp
- Page 759 and 760:
egions of the world. In addition to
- Page 761 and 762:
50-55% fat. Production figures for
- Page 763 and 764:
utter with up to 5% of another fat
- Page 765 and 766:
market with high overrun and good s
- Page 767 and 768:
11. D. Hettinga. Butter. In: Bailey
- Page 769 and 770:
25 Lipid Biotechnology KUMAR D. MUK
- Page 771 and 772:
Table 1 Lipid Content and Levels of
- Page 773 and 774:
as 80% lipids of which about 90% ar
- Page 775 and 776:
Table 6 Wax Esters Formed by Acinet
- Page 777 and 778:
Some other biosurfactants include e
- Page 779 and 780:
Figure 6 Microbial production of hy
- Page 781 and 782:
Figure 9 Microbial production of ke
- Page 783 and 784:
Figure 12 Microbial production of k
- Page 785 and 786:
Table 8 Specificity of Triacylglyce
- Page 787 and 788:
Figure 16 Lipase-catalyzed transest
- Page 789 and 790:
Figure 20 Preparation of structured
- Page 791 and 792:
Figure 24 Preparation of monoacylgl
- Page 793 and 794:
Fatty acid esters of polyols are us
- Page 795 and 796:
Figure 29 Specificity constants in
- Page 797 and 798:
Figure 31 Preparation of concentrat
- Page 799 and 800:
Figure 35 Enrichment of very long c
- Page 801 and 802:
B. Phospholipases Figure 38 shows t
- Page 803 and 804:
Lysophosphatidic acid has been prep
- Page 805 and 806:
Figure 41 Transesterification of ph
- Page 807 and 808:
Figure 46 Enzymatic production of h
- Page 809 and 810:
Figure 50 Hydration of linoleic aci
- Page 811 and 812:
Figure 51 Principle of enzymatic de
- Page 813 and 814:
13. E. Molina Grima, J. A. Sánchez
- Page 815 and 816:
53. M. Powalla, S. Lang, and V. Wra
- Page 817 and 818:
96. R. Schuch and K. D. Mukherjee.
- Page 819 and 820:
138. K. D. Mukherjee and I. Kiewitt
- Page 821 and 822:
179. U. T. Bornscheuer, H. Stamatis
- Page 823 and 824:
222. H. Stamatis, V. Sereti, and F.
- Page 825 and 826:
265. S. R. Moore and G. P. McNeill.
- Page 827 and 828:
303. C. Virto, I. Svensson, and P.
- Page 829 and 830:
343. E. Blee and F. Schuber. Regio-
- Page 831 and 832:
26 Microbial Lipases JOHN D. WEETE
- Page 833 and 834:
ation. Fungal lipases typically exi
- Page 835 and 836:
of buffer A containing 1 M ammonium
- Page 837 and 838:
without shaking for 30 minutes, whe
- Page 839 and 840:
the C domain of the protein through
- Page 841 and 842:
Figure 2 (Continued) Figure 3 Schem
- Page 843 and 844:
on the substrate and presence or ab
- Page 845 and 846:
Table 3 Selectivities of Multiple E
- Page 847 and 848:
lanuginosa, C. antarctica B, Rhizop
- Page 849 and 850:
10. C. T. Hou and T. M. Johnston. S
- Page 851 and 852:
53. C. C. Akoh. Enzymatic synthesis
- Page 853 and 854:
94. D. M. Lawson, A. M. Brzozowski,
- Page 855 and 856:
135. B. K. Yang and J. P. Chen. Gel
- Page 857 and 858:
27 Enzymatic Interesterification WE
- Page 859 and 860:
esterification of butterfat at 40
- Page 861 and 862:
also found to decrease the crystall
- Page 863 and 864:
T c than animal fats. The T c for v
- Page 865 and 866:
in the oxyanion hole is the amino a
- Page 867 and 868:
of the interface as a measure of su
- Page 869 and 870:
Figure 11 Catalytic mechanism for l
- Page 871 and 872:
Figure 13 Triacylglycerol products
- Page 873 and 874:
imization of interesterification, a
- Page 875 and 876:
supports include high losses of act
- Page 877 and 878:
is a thin layer located directly ne
- Page 879 and 880:
volume per year. The volumetric act
- Page 881 and 882:
and removal of reactants and produc
- Page 883 and 884:
vinyl chloride. In a membrane such
- Page 885 and 886:
of the enzyme. Animal and plant lip
- Page 887 and 888:
esters as surface active agents dur
- Page 889 and 890:
14. P. Kalo, H. Huotari, and M. Ant
- Page 891 and 892:
57. F. Pabai, S. Kermasha, and A. M
- Page 893 and 894:
94. J. Kurashige. Enzymatic convers
- Page 895 and 896:
28 Structured Lipids CASIMIR C. AKO
- Page 897 and 898:
Figure 2 Structure of a physical mi
- Page 899 and 900:
2. Medium Chain Fatty Acids and Tri
- Page 901 and 902:
Figure 4 Pathway for eicosanoid bio
- Page 903 and 904:
leukotrienes (hydroxy fatty acids a
- Page 905 and 906:
Figure 7 Structure of Benefat (bran
- Page 907 and 908:
cause of the huge capital investmen
- Page 909 and 910:
Figure 8 Reaction scheme showing ac
- Page 911 and 912:
alters the native conformation of t
- Page 913 and 914:
Table 6 Advantages of Enzymatic App
- Page 915 and 916:
Figure 13 Stereochemical configurat
- Page 917 and 918:
IV. NUTRITIONAL AND MEDICAL APPLICA
- Page 919 and 920:
Table 9 Factors That Affect Outlook
- Page 921 and 922:
27. G. O. Burr and M. D. Burr. A ne
- Page 923 and 924:
69. M. Reslow, P. Aldercreutz, and
- Page 925 and 926:
113. C. J. Gollaher, E. S. Swenson,
- Page 927 and 928:
29 Biosynthesis of Fatty Acids and
- Page 929 and 930:
olive (Olea europea), and avocado (
- Page 931 and 932:
Table 2 (Continued) Fatty acid a Sp
- Page 933 and 934:
2. Basic Features The functional un
- Page 935 and 936:
Hydroxy-Acyl ACP dehydrase (Crotony
- Page 937 and 938:
Figure 1 Steps of fatty acid biosyn
- Page 939 and 940:
The three KAS isoforms are assigned
- Page 941 and 942:
chain length acyl-ACP residues, and
- Page 943 and 944:
10-carbon and 14- to 16-carbon acyl
- Page 945 and 946:
In developing castor seed, BC and A
- Page 947 and 948:
(viz., seed, fruit) genes, and this
- Page 949 and 950:
Figure 4 Fatty acid modification re
- Page 951 and 952:
12-MO, the cDNA for the enzyme has
- Page 953 and 954:
Table 5 Enzyme Activities Involved
- Page 955 and 956:
of reactivity is also supported by
- Page 957 and 958:
tems, PTAP is a likely candidate fo
- Page 959 and 960:
ifying 18:1 �9 and deacylating 18
- Page 961 and 962:
oils (rich in 18:3 �6,9,12), and
- Page 963 and 964:
eticulum subpopulations also finds
- Page 965 and 966:
CPT (DAG ↔ PC) Extensive involvem
- Page 967 and 968:
available (cf. Sec. V.C.4 and Ref.
- Page 969 and 970:
erol backbone of triacylglycerols c
- Page 971 and 972:
14. E. Heinz. Biosynthesis of polyu
- Page 973 and 974:
56. R. C. Clough, A. L. Matthis, S.
- Page 975 and 976:
91. R. J. Heath and C. O. Rock. Eno
- Page 977 and 978:
124. R. Schuch, F. M. Brück, M. Br
- Page 979 and 980:
Kader and P. Mazliak, eds.). Kluwer
- Page 981 and 982:
197. Y. Cao, K. Oo, and A. H. C. Hu
- Page 983 and 984:
241. M. C. Dobarganes, G. Márquez-
- Page 985 and 986:
30 Genetic Engineering of Crops Tha
- Page 987 and 988:
existing genes in the host plant ge
- Page 989 and 990:
It becomes the plant breeder’s jo
- Page 991 and 992:
previously mentioned low-linolenic
- Page 993 and 994:
develop and apply methods, evaluate
- Page 995 and 996:
enzyme with high activity for placi
- Page 997 and 998:
activity in the oil palm mesocarp s
- Page 999 and 1000:
Figure 4 Commercial applications of
- Page 1001 and 1002:
and mildness. Because the perennial
- Page 1003 and 1004:
In the context of developing increa
- Page 1005 and 1006:
occurs esterified at the sn-2 posit
- Page 1007 and 1008:
as high as coconut or palm kernel o
- Page 1009 and 1010:
systems. Key to assessing the oppor
- Page 1011 and 1012:
property encouraged us to look for
- Page 1013 and 1014:
mercial lauric fats based on both P