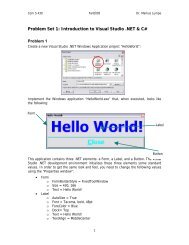
Workshop proceeding - final.pdf - Faculty of Information and ...
Workshop proceeding - final.pdf - Faculty of Information and ...
Workshop proceeding - final.pdf - Faculty of Information and ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
triphenyltetrazolium chloride (TTC) solution (1%) for 30 min in phosphate buffer at 37℃. Sections<br />
were fixed overnight in 4% paraformaldehyde for contrast enhancement between stained <strong>and</strong><br />
unstained tissue. TTC stained the viable tissue with red, while the necrotic tissue remained discolored.<br />
The sections were then placed between two cover slips <strong>and</strong> digitally photographed using a Nikon<br />
coolpix S10 camera, <strong>and</strong> quantified with the weight respectively. The area <strong>of</strong> irreversible injury (TTC<br />
negative) is presented as a percentage <strong>of</strong> the area(the irreversible injury area/the total weight <strong>of</strong><br />
ventricles).<br />
2.7.Measured the density <strong>of</strong> capillary<br />
The streptavidin peroxidase (SP) immunohistochemical method was used to detect the expression<br />
<strong>of</strong> factor VIII in myocardial infarction margin areas. The dilution <strong>of</strong> factor VIII rat monoclonal<br />
antibody (Santa Cruz, CA, USA) was 1:100. The procedure was performed according to the<br />
manufacturer’s instructions.The positive cells were identified, counted <strong>and</strong> analyzed under the<br />
inverted phase-contrast microscope (Olympus, Japan) in 3 different fields(0.1mm 2 ) at 400<br />
magnification using Image-proplus 6.0 s<strong>of</strong>tware.Results were normalized by arbitrarily setting the<br />
total tube area <strong>of</strong> control to 100%.<br />
2.8. Real time fluorescent quantitation PCR (RT-PCR)<br />
After ligation, myocardial tissues were harvested at the times indicated, washed twice with icecold<br />
phosphate-buffered saline (PBS) <strong>and</strong> collected by centrifugation. Total RNA was isolated using<br />
the Trizol reagent (MRC, USA) according to the manufacturer's instructions. Total RNA (5 µL) was<br />
converted to complementary DNA (cDNA) using Revert Aid First Str<strong>and</strong> cDNA Synthesis Kit. A<br />
5µL aliquot <strong>of</strong> the resulting cDNA was used as template for PCR amplification with the following<br />
primers: mini-TrpRS, P1 (forward, 5’- CCC TGC TGC ACT CCA CCT T-3’), P2 (reverse, 5’- ACG<br />
CAT GCT TAT TGA CCT TG-3’); mini- TyrRS, P3 (forward, 5’- CAT CTG ATG AAT CCT ATG<br />
GTT -3’), P4 (reverse, 5’- GGA TCA CAA ACT CGG ACT TA-3’); β-actin, P5 (forward, 5’-GCC<br />
AAC ACA GTG CTG TCT -3’), P6 (reverse, 5’-AGG AGC AAT GAT CTT GAT CTT -3’). The<br />
amplifications were performed by an initial denaturation (94 °C for 2 min), followed by 45 cycles <strong>of</strong><br />
denaturation, annealing <strong>and</strong> extension (94 °C for 20 s, 54 °C for 20 s, 72 °C for 30 s), <strong>and</strong> a <strong>final</strong><br />
extension (72 °C for 5 min). The transcript <strong>of</strong> β-actin was also amplified by RT-PCR from the same<br />
cDNA template <strong>and</strong> was used as an internal control. All the primers were designed <strong>and</strong> synthesized by<br />
Genepharma (Shanghai, China). The identity <strong>of</strong> each PCR product was confirmed by DNA sequencing.<br />
The pictures were scanned <strong>and</strong> analyzed by the Gel Doc 1000 gel imaging system.<br />
2.9.Statistical analysis<br />
Results are expressed as mean ± st<strong>and</strong>ard deviation. Comparison <strong>of</strong> means was performed by<br />
means <strong>of</strong> the analysis <strong>of</strong> variance procedure (Student–Newman–keuls test, SPSS 13.0 for Windows).<br />
P