IGCAR : Annual Report - Indira Gandhi Centre for Atomic Research
IGCAR : Annual Report - Indira Gandhi Centre for Atomic Research
IGCAR : Annual Report - Indira Gandhi Centre for Atomic Research
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
IGC<br />
<strong>Annual</strong> <strong>Report</strong> 2007<br />
f 1<br />
(dl/l) PAC<br />
f 2<br />
f 3<br />
f 4<br />
1.0<br />
0.8<br />
0.6<br />
0.4<br />
0.5<br />
0.4<br />
0.3<br />
0.2<br />
0.1<br />
0.2<br />
0.04<br />
0.02<br />
0.00<br />
-0.02<br />
-0.04<br />
0.4<br />
0.3<br />
0.2<br />
0.1<br />
240 280 320 360 400 440<br />
Temperature (K)<br />
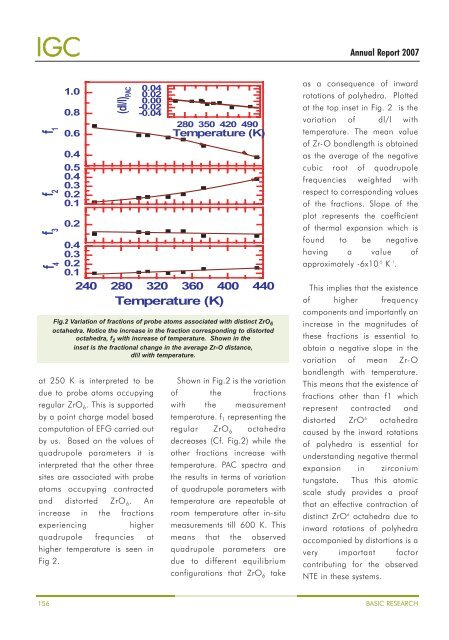
at 250 K is interpreted to be<br />
due to probe atoms occupying<br />
regular ZrO 6 . This is supported<br />
by a point charge model based<br />
computation of EFG carried out<br />
by us. Based on the values of<br />
quadrupole parameters it is<br />
interpreted that the other three<br />
sites are associated with probe<br />
atoms occupying contracted<br />
and distorted ZrO 6 . An<br />
increase in the fractions<br />
experiencing<br />
higher<br />
quadrupole frequncies at<br />
higher temperature is seen in<br />
Fig 2.<br />
280 350 420 490<br />
Temperature (K)<br />
Fig.2 Variation of fractions of probe atoms associated with distinct ZrO 6<br />
octahedra. Notice the increase in the fraction corresponding to distorted<br />
octahedra, f 2 with increase of temperature. Shown in the<br />
inset is the fractional change in the average Zr-O distance,<br />
dl/l with temperature.<br />
Shown in Fig.2 is the variation<br />
of the fractions<br />
with the measurement<br />
temperature. f 1 representing the<br />
regular ZrO 6 octahedra<br />
decreases (Cf. Fig.2) while the<br />
other fractions increase with<br />
temperature. PAC spectra and<br />
the results in terms of variation<br />
of quadrupole parameters with<br />
temperature are repeatable at<br />
room temperature after in-situ<br />
measurements till 600 K. This<br />
means that the observed<br />
quadrupole parameters are<br />
due to different equilibrium<br />
configurations that ZrO 6 take<br />
as a consequence of inward<br />
rotations of polyhedra. Plotted<br />
at the top inset in Fig. 2 is the<br />
variation of dl/l with<br />
temperature. The mean value<br />
of Zr-O bondlength is obtained<br />
as the average of the negative<br />
cubic root of quadrupole<br />
frequencies weighted with<br />
respect to corresponding values<br />
of the fractions. Slope of the<br />
plot represents the coefficient<br />
of thermal expansion which is<br />
found to be negative<br />
having a value of<br />
approximately -6x10 -5 K -1 .<br />
This implies that the existence<br />
of higher frequency<br />
components and importantly an<br />
increase in the magnitudes of<br />
these fractions is essential to<br />
obtain a negative slope in the<br />
variation of mean Zr-O<br />
bondlength with temperature.<br />
This means that the existence of<br />
fractions other than f1 which<br />
represent contracted and<br />
distorted ZrO 6 octahedra<br />
caused by the inward rotations<br />
of polyhedra is essential <strong>for</strong><br />
understanding negative thermal<br />
expansion in zirconium<br />
tungstate. Thus this atomic<br />
scale study provides a proof<br />
that an effective contraction of<br />
distinct ZrO 6 octahedra due to<br />
inward rotations of polyhedra<br />
accompanied by distortions is a<br />
very important factor<br />
contributing <strong>for</strong> the observed<br />
NTE in these systems.<br />
156 BASIC RESEARCH