Clinical Trials and Preclinical Infrastructure Asset Map - Life Sciences
Clinical Trials and Preclinical Infrastructure Asset Map - Life Sciences
Clinical Trials and Preclinical Infrastructure Asset Map - Life Sciences
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
CLINICAL TRIALS AND PRECLINICAL INFRASTRUCTURE ASSET MAP<br />
9<br />
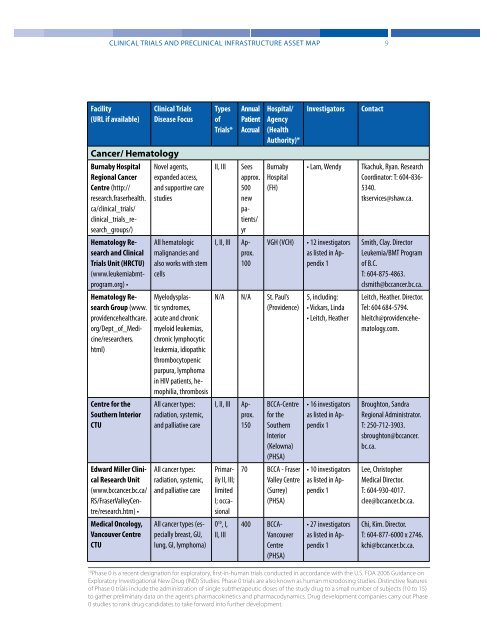
Facility<br />
(URL if available)<br />
<strong>Clinical</strong> <strong>Trials</strong><br />
Disease Focus<br />
Types<br />
of<br />
<strong>Trials</strong>*<br />
Annual<br />
Patient<br />
Accrual<br />
Hospital/<br />
Agency<br />
(Health<br />
Authority)*<br />
Investigators<br />
Contact<br />
Cancer/ Hematology<br />
Burnaby Hospital<br />
Regional Cancer<br />
Centre (http://<br />
research.fraserhealth.<br />
ca/clinical_trials/<br />
clinical_trials_research_groups/)<br />
Novel agents,<br />
exp<strong>and</strong>ed access,<br />
<strong>and</strong> supportive care<br />
studies<br />
II, III<br />
Sees<br />
approx.<br />
500<br />
new<br />
patients/<br />
yr<br />
Burnaby<br />
Hospital<br />
(FH)<br />
• Lam, Wendy<br />
Tkachuk, Ryan. Research<br />
Coordinator: T: 604-836-<br />
5340.<br />
tkservices@shaw.ca.<br />
Hematology Research<br />
<strong>and</strong> <strong>Clinical</strong><br />
<strong>Trials</strong> Unit (HRCTU)<br />
(www.leukemiabmtprogram.org)<br />
•<br />
All hematologic<br />
malignancies <strong>and</strong><br />
also works with stem<br />
cells<br />
I, II, III Approx.<br />
100<br />
VGH (VCH)<br />
• 12 investigators<br />
as listed in Appendix<br />
1<br />
Smith, Clay. Director<br />
Leukemia/BMT Program<br />
of B.C.<br />
T: 604-875-4863.<br />
clsmith@bccancer.bc.ca.<br />
Hematology Research<br />
Group (www.<br />
providencehealthcare.<br />
org/Dept_of_Medicine/researchers.<br />
html)<br />
Myelodysplastic<br />
syndromes,<br />
acute <strong>and</strong> chronic<br />
myeloid leukemias,<br />
chronic lymphocytic<br />
leukemia, idiopathic<br />
thrombocytopenic<br />
purpura, lymphoma<br />
in HIV patients, hemophilia,<br />
thrombosis<br />
N/A N/A St. Paul’s<br />
(Providence)<br />
5, including:<br />
• Vickars, Linda<br />
• Leitch, Heather<br />
Leitch, Heather. Director.<br />
Tel: 604 684-5794.<br />
hleitch@providencehematology.com.<br />
Centre for the<br />
Southern Interior<br />
CTU<br />
All cancer types:<br />
radiation, systemic,<br />
<strong>and</strong> palliative care<br />
I, II, III Approx.<br />
150<br />
BCCA-Centre<br />
for the<br />
Southern<br />
Interior<br />
(Kelowna)<br />
(PHSA)<br />
• 16 investigators<br />
as listed in Appendix<br />
1<br />
Broughton, S<strong>and</strong>ra<br />
Regional Administrator.<br />
T: 250-712-3903.<br />
sbroughton@bccancer.<br />
bc.ca.<br />
Edward Miller <strong>Clinical</strong><br />
Research Unit<br />
(www.bccancer.bc.ca/<br />
RS/FraserValleyCentre/research.htm)<br />
•<br />
All cancer types:<br />
radiation, systemic,<br />
<strong>and</strong> palliative care<br />
Primarily<br />
II, III;<br />
limited<br />
I; occasional<br />
70 BCCA - Fraser<br />
Valley Centre<br />
(Surrey)<br />
(PHSA)<br />
• 10 investigators<br />
as listed in Appendix<br />
1<br />
Lee, Christopher<br />
Medical Director.<br />
T: 604-930-4017.<br />
clee@bccancer.bc.ca.<br />
Medical Oncology,<br />
Vancouver Centre<br />
CTU<br />
All cancer types (especially<br />
breast, GU,<br />
lung, GI, lymphoma)<br />
0 10 , I,<br />
II, III<br />
400 BCCA-<br />
Vancouver<br />
Centre<br />
(PHSA)<br />
• 27 investigators<br />
as listed in Appendix<br />
1<br />
Chi, Kim. Director.<br />
T: 604-877-6000 x 2746.<br />
kchi@bccancer.bc.ca.<br />
10<br />
Phase 0 is a recent designation for exploratory, first-in-human trials conducted in accordance with the U.S. FDA 2006 Guidance on<br />
Exploratory Investigational New Drug (IND) Studies. Phase 0 trials are also known as human microdosing studies. Distinctive features<br />
of Phase 0 trials include the administration of single subtherapeutic doses of the study drug to a small number of subjects (10 to 15)<br />
to gather preliminary data on the agent’s pharmacokinetics <strong>and</strong> pharmacodynamics. Drug development companies carry out Phase<br />
0 studies to rank drug c<strong>and</strong>idates to take forward into further development.