Clinical Trials and Preclinical Infrastructure Asset Map - Life Sciences
Clinical Trials and Preclinical Infrastructure Asset Map - Life Sciences
Clinical Trials and Preclinical Infrastructure Asset Map - Life Sciences
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
CLINICAL TRIALS AND PRECLINICAL INFRASTRUCTURE ASSET MAP<br />
265<br />
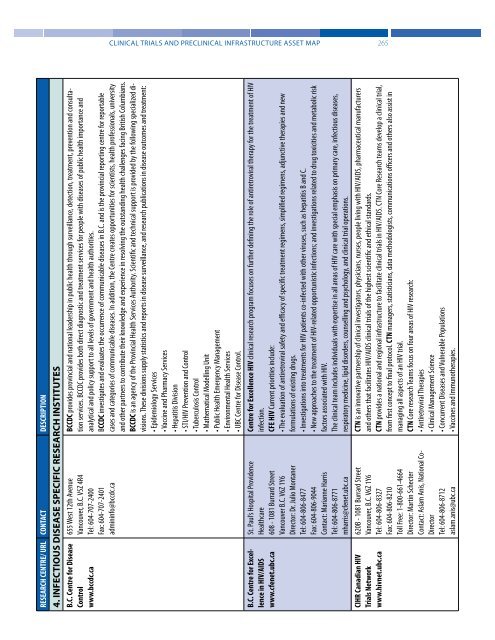
RESEARCH CENTRE/ URL CONTACT DESCRIPTION<br />
4. INFECTIOUS DISEASE SPECIFIC RESEARCH INSTITUTES<br />
B.C. Centre for Disease<br />
Control<br />
www.bccdc.ca<br />
B.C. Centre for Excellence<br />
in HIV/AIDS<br />
www.cfenet.ubc.ca<br />
CIHR Canadian HIV<br />
<strong>Trials</strong> Network<br />
www.hivnet.ubc.ca<br />
655 West 12th Avenue<br />
Vancouver, B.C. V5Z 4R4<br />
Tel: 604-707-2400<br />
Fax: 604-707-2401<br />
admininfo@bccdc.ca<br />
St. Paul’s Hospital Providence<br />
Healthcare<br />
608 - 1081 Burrard Street<br />
Vancouver B.C. V6Z 1Y6<br />
Director: Dr. Julio Montaner<br />
Tel: 604-806-8477<br />
Fax: 604-806-9044<br />
Contact: Marianne Harris<br />
Tel: 604-806-8771<br />
mharris@cfenet.ubc.ca<br />
620B - 1081 Burrard Street<br />
Vancouver, B.C. V6Z 1Y6<br />
Tel: 604-806-8327<br />
Fax: 604-806-8210<br />
Toll Free: 1-800-661-4664<br />
Director: Martin Schecter<br />
Contact: Aslam Anis, National Co-<br />
Director<br />
Tel: 604-806-8712<br />
aslam.anis@ubc.ca<br />
BCCDC provides provincial <strong>and</strong> national leadership in public health through surveillance, detection, treatment, prevention <strong>and</strong> consultation<br />
services. BCCDC provides both direct diagnostic <strong>and</strong> treatment services for people with diseases of public health importance <strong>and</strong><br />
analytical <strong>and</strong> policy support to all levels of government <strong>and</strong> health authorities.<br />
BCCDC investigates <strong>and</strong> evaluates the occurrence of communicable diseases in B.C. <strong>and</strong> is the provincial reporting centre for reportable<br />
cases <strong>and</strong> categories of communicable diseases. In addition, the Centre creates opportunities for scientists, health professionals, university<br />
<strong>and</strong> other partners to contribute their knowledge <strong>and</strong> experience in resolving the outst<strong>and</strong>ing health challenges facing British Columbians.<br />
BCCDC is an agency of the Provincial Health Services Authority. Scientific <strong>and</strong> technical support is provided by the following specialized divisions.<br />
These divisions supply statistics <strong>and</strong> reports in disease surveillance, <strong>and</strong> research publications in disease outcomes <strong>and</strong> treatment:<br />
• Epidemiology Services<br />
• Vaccine <strong>and</strong> Pharmacy Services<br />
• Hepatitis Division<br />
• STI/HIV Prevention <strong>and</strong> Control<br />
• Tuberculosis Control<br />
• Mathematical Modelling Unit<br />
• Public Health Emergency Management<br />
• Environmental Health Services<br />
• UBC Centre for Disease Control.<br />
Centre for Excellence HIV clinical research program focuses on further defining the role of antiretroviral therapy for the treatment of HIV<br />
infection.<br />
CFE HIV Current priorities include:<br />
• The evaluation of antiretroviral safety <strong>and</strong> efficacy of specific treatment regimens, simplified regimens, adjunctive therapies <strong>and</strong> new<br />
formulations of existing drugs.<br />
• Investigations into treatments for HIV patients co-infected with other viruses, such as hepatitis B <strong>and</strong> C.<br />
• New approaches to the treatment of HIV-related opportunistic infections; <strong>and</strong> investigations related to drug toxicities <strong>and</strong> metabolic risk<br />
factors associated with HIV.<br />
The clinical team includes individuals with expertise in all areas of HIV care with special emphasis on primary care, infectious diseases,<br />
respiratory medicine, lipid disorders, counselling <strong>and</strong> psychology, <strong>and</strong> clinical trial operations.<br />
CTN is an innovative partnership of clinical investigators, physicians, nurses, people living with HIV/AIDS, pharmaceutical manufacturers<br />
<strong>and</strong> others that facilitates HIV/AIDS clinical trials of the highest scientific <strong>and</strong> ethical st<strong>and</strong>ards.<br />
CTN provides a national <strong>and</strong> regional infrastructure to facilitate clinical trials in HIV/AIDS. CTN Core Research teams develop a clinical trial,<br />
from first concept to final protocol. CTN managers, statisticians, data methodologists, communications officers <strong>and</strong> others also assist in<br />
managing all aspects of an HIV trial.<br />
CTN Core research Teams focus on four areas of HIV research:<br />
• Antiretroviral Therapies<br />
• <strong>Clinical</strong> Management Science<br />
• Concurrent Diseases <strong>and</strong> Vulnerable Populations<br />
• Vaccines <strong>and</strong> Immunotherapies.