Clinical Trials and Preclinical Infrastructure Asset Map - Life Sciences
Clinical Trials and Preclinical Infrastructure Asset Map - Life Sciences
Clinical Trials and Preclinical Infrastructure Asset Map - Life Sciences
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
22<br />
BRITISH COLUMBIA<br />
Much clinical research - particularly cancer-related - is multi-centred <strong>and</strong> collaborative, thus subject to<br />
ethical review by each of the involved institutions. To minimize the requirement for multiple reviews<br />
of research presented to the UBC, Providence, <strong>and</strong> BCCA REBs, these institutions have agreed that the<br />
initial review may be conducted by any of their four REBs <strong>and</strong> that the other three will, in most cases,<br />
honour the outcome of the that review. Further, the REB that reviews <strong>and</strong> approves a protocol is responsible<br />
for subsequent supervision.<br />
The MSFHR-led “B.C. Ethics Harmonization Initiative” 14 is developing a harmonized provincial approach<br />
to ethics approval, including:<br />
• Creating common forms (e.g. application forms, informed consent)<br />
• Developing a shared/ common IT platform <strong>and</strong> tools accessible to researchers <strong>and</strong> institutions at<br />
which human subject research is undertaken in B.C.<br />
• Exploring how ethics review for multi-centre trials can be more efficient, consistent, <strong>and</strong> timely,<br />
possibly with some degree of inter-institutional reciprocity<br />
• Developing common educational <strong>and</strong> training resources to be shared by REBs.<br />
Training Facilities<br />
All B.C.’s CTUs, centres, <strong>and</strong> study sites ensure that their investigators <strong>and</strong> staff are trained in clinical trial<br />
procedures, SOPs, <strong>and</strong> appropriate (i.e., Health Canada, FDA, ICH) GCP regulations <strong>and</strong> guidelines, as<br />
indicated in Appendix 1. Such training is usually in-house or via on-line GCP courses. In addition, there<br />
are at least nine training programs in the Province that specifically focus on clinical trials. These are listed<br />
in Table 4 <strong>and</strong> detailed in Appendix 5.<br />
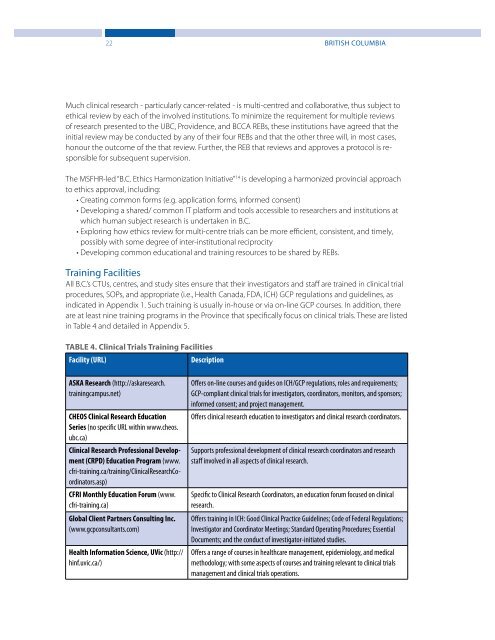
TABLE 4. <strong>Clinical</strong> <strong>Trials</strong> Training Facilities<br />
Facility (URL)<br />
Description<br />
ASKA Research (http://askaresearch.<br />
trainingcampus.net)<br />
CHEOS <strong>Clinical</strong> Research Education<br />
Series (no specific URL within www.cheos.<br />
ubc.ca)<br />
<strong>Clinical</strong> Research Professional Development<br />
(CRPD) Education Program (www.<br />
cfri-training.ca/training/<strong>Clinical</strong>ResearchCoordinators.asp)<br />
CFRI Monthly Education Forum (www.<br />
cfri-training.ca)<br />
Global Client Partners Consulting Inc.<br />
(www.gcpconsultants.com)<br />
Health Information Science, UVic (http://<br />
hinf.uvic.ca/)<br />
Offers on-line courses <strong>and</strong> guides on ICH/GCP regulations, roles <strong>and</strong> requirements;<br />
GCP-compliant clinical trials for investigators, coordinators, monitors, <strong>and</strong> sponsors;<br />
informed consent; <strong>and</strong> project management.<br />
Offers clinical research education to investigators <strong>and</strong> clinical research coordinators.<br />
Supports professional development of clinical research coordinators <strong>and</strong> research<br />
staff involved in all aspects of clinical research.<br />
Specific to <strong>Clinical</strong> Research Coordinators, an education forum focused on clinical<br />
research.<br />
Offers training in ICH: Good <strong>Clinical</strong> Practice Guidelines; Code of Federal Regulations;<br />
Investigator <strong>and</strong> Coordinator Meetings; St<strong>and</strong>ard Operating Procedures; Essential<br />
Documents; <strong>and</strong> the conduct of investigator-initiated studies.<br />
Offers a range of courses in healthcare management, epidemiology, <strong>and</strong> medical<br />
methodology; with some aspects of courses <strong>and</strong> training relevant to clinical trials<br />
management <strong>and</strong> clinical trials operations.