Invasive breast carcinoma - IARC
Invasive breast carcinoma - IARC
Invasive breast carcinoma - IARC
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Synonyms<br />
Ductal intraepithelial neoplasia 1A (DIN<br />
1A); clinging <strong>carcinoma</strong>, monomorphous<br />
type; atypical cystic lobules; atypical lobules,<br />
type A; atypical columnar change.<br />
Risk of progression<br />
Some cases of flat epithelial atypia may<br />
progress to invasive <strong>breast</strong> cancer but<br />
no quantitative epidemiological data are<br />
currently available for risk estimation.<br />
Histopathology<br />
A flat type of epithelial atypia, this<br />
change is characterized by replacement<br />
of the native epithelial cells by a single<br />
layer of mildly atypical cells often with<br />
apical snouts, or proliferation of a monotonous<br />
atypical cell population in the<br />
form of stratification of uniform, cuboidal<br />
to columnar cells generally up to 3-5 cell<br />
layers with occasional mounding.<br />
Arcades and micropapillary formations<br />
a re absent or very rare. The TDLUs<br />
involved are variably distended and may<br />
contain secretory or floccular material<br />
that often contains microcalcifications.<br />
Genetic alterations<br />
Data on genetic alterations in flat<br />
epithelial atypia are limited. LOH has<br />
been found in at least one locus in 70%<br />
of cases in a study evaluating eight loci<br />
in thirteen lesions {1889}. LOH on 11q<br />
(D11S1311) was the most commonly<br />
noted in 50% of the pure flat atypia,<br />
Fig. 1.82 Flat epithelial atypia. Immunostain for<br />
CK34βE12 shows no staining in the neoplastic cells<br />
lining the ductules, but the residual luminal epithelial<br />
cells adherent along the luminal surface show<br />
intense staining.<br />
while among seven flat atypias associated<br />
with infiltrating <strong>carcinoma</strong>s, the frequency<br />
of LOH on 11q (D11S1311) was<br />
57% {1889}.<br />
Atypical ductal hyperplasia<br />
(ADH)<br />
Definition<br />
A neoplastic intraductal lesion characterized<br />
by proliferation of evenly distributed,<br />
monomorphic cells and associated with<br />
a moderately elevated risk for progression<br />
to invasive <strong>breast</strong> cancer.<br />
Synonyms<br />
Ductal intraepithelial neoplasia 1B (DIN<br />
1B), atypical intraductal hyperplasia.<br />
Risk of progression<br />
The Cancer Committee of the College of<br />
American Pathologists has assigned<br />
ADH a moderately increased risk (RR of<br />
4.0-5.0) for subsequent development of<br />
invasive <strong>breast</strong> cancer {885}. Following<br />
a <strong>breast</strong> biopsy diagnosis of ADH, 3.7-<br />
22% of the women develop invasive<br />
c a rcinomas {299,733,1520,2886}. On<br />
the other hand, ADH has also been<br />
p resent in 2.2% {2158} to 10.5% {1688}<br />
of controls who did not develop subsequent<br />
<strong>carcinoma</strong>. The average interval<br />
to the subsequent development of invasive<br />
<strong>carcinoma</strong> is 8.3 years compare d<br />
to 14.3 years for women with UDH<br />
{ 2 8 8 6 } .<br />
However, drastically different relative risk<br />
(RR) estimations have been reported for<br />
ADH, ranging from a low of 2.4 to a high<br />
of 13 {412,732,1688,1775,1830,2155,<br />
2158}. The upper values are even higher<br />
than the RR of 8-11 suggested for DCIS<br />
{732,885}. On the other hand, the RR of<br />
2.4 for ADH reported in one study {1775}<br />
is much closer to the RR of 1.9 associated<br />
with UDH.<br />
Histopathology<br />
The most distinctive feature of this lesion<br />
is the proliferation of evenly distributed,<br />
monomorphic cells with generally ovoid<br />
to rounded nuclei. The cells may grow in<br />
m i c ropapillae, tufts, fronds, arc a d e s ,<br />
rigid bridges, solid and cribriform patterns.<br />
Cytologically, ADH corresponds to<br />
low grade DCIS.<br />
ADH is diagnosed when characteristic<br />
cells coexist with patterns of UDH,<br />
and/or there is partial involvement of<br />
TDLU by classic morphology. There is<br />
c u r rently no general agreement on<br />
whether quantitative criteria should be<br />
applied to separate ADH from low grade<br />
DCIS. Some define the upper limit of<br />
ADH as one or more completely involved<br />
duct/ductular cross sections measuring<br />
≤2 mm in aggregate, while others require<br />
that the characteristic cytology and<br />
architecture be present completely in two<br />
spaces. Microcalcifications may be<br />
absent, focal or extensive within the<br />
lumen of involved ducts; its presence<br />
does not impact diagnosis.<br />
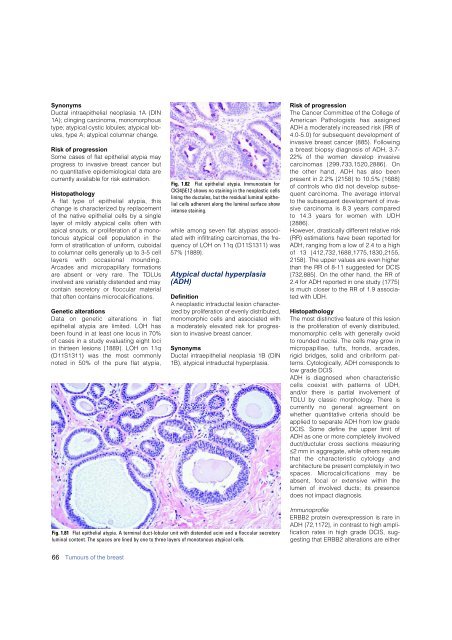
Fig. 1.81 Flat epithelial atypia. A terminal duct-lobular unit with distended acini and a floccular secretory<br />
luminal content. The spaces are lined by one to three layers of monotonous atypical cells.<br />
Immunoprofile<br />
ERBB2 protein overexpression is rare in<br />
ADH {72,1172}, in contrast to high amplification<br />
rates in high grade DCIS, suggesting<br />
that ERBB2 alterations are either<br />
66 Tumours of the <strong>breast</strong>