Dictionary of Evidence-based Medicine.pdf
Dictionary of Evidence-based Medicine.pdf
Dictionary of Evidence-based Medicine.pdf
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Dictionary</strong> <strong>of</strong> <strong>Evidence</strong>-<strong>based</strong> <strong>Medicine</strong> 123<br />
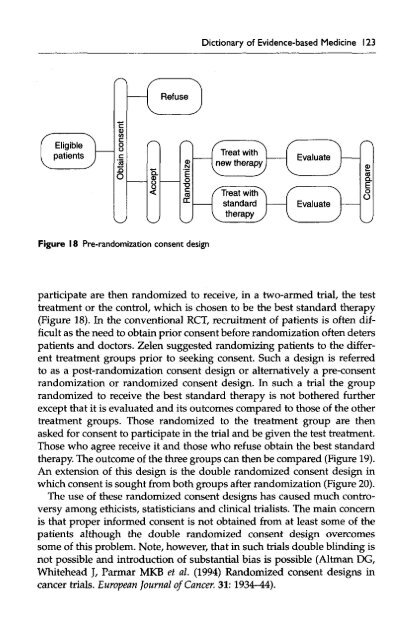
Figure 18 Pre-randomization consent design<br />
participate are then randomized to receive, in a two-armed trial, the test<br />
treatment or the control, which is chosen to be the best standard therapy<br />
(Figure 18). In the conventional RCT, recruitment <strong>of</strong> patients is <strong>of</strong>ten difficult<br />
as the need to obtain prior consent before randomization <strong>of</strong>ten deters<br />
patients and doctors. Zelen suggested randomizing patients to the different<br />
treatment groups prior to seeking consent. Such a design is referred<br />
to as a post-randomization consent design or alternatively a pre-consent<br />
randomization or randomized consent design. In such a trial the group<br />
randomized to receive the best standard therapy is not bothered further<br />
except that it is evaluated and its outcomes compared to those <strong>of</strong> the other<br />
treatment groups. Those randomized to the treatment group are then<br />
asked for consent to participate in the trial and be given the test treatment.<br />
Those who agree receive it and those who refuse obtain the best standard<br />
therapy. The outcome <strong>of</strong> the three groups can then be compared (Figure 19).<br />
An extension <strong>of</strong> this design is the double randomized consent design in<br />
which consent is sought from both groups after randomization (Figure 20).<br />
The use <strong>of</strong> these randomized consent designs has caused much controversy<br />
among ethicists, statisticians and clinical trialists. The main concern<br />
is that proper informed consent is not obtained from at least some <strong>of</strong> the<br />
patients although the double randomized consent design overcomes<br />
some <strong>of</strong> this problem. Note, however, that in such trials double blinding is<br />
not possible and introduction <strong>of</strong> substantial bias is possible (Altman DG,<br />
Whitehead J, Parmar MKB et al. (1994) Randomized consent designs in<br />
cancer trials. European Journal <strong>of</strong> Cancer. 31:1934-44).