- Page 2 and 3:
Multidisciplinaire richtlijnGuillai
- Page 4 and 5:
Multidisciplinaire richtlijn Guilla
- Page 6 and 7:
Multidisciplinaire richtlijn Guilla
- Page 8 and 9:
Multidisciplinaire richtlijn Guilla
- Page 10 and 11:
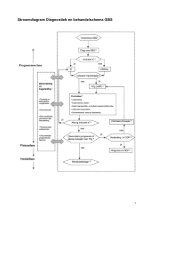
Stroomdiagram Diagnostiek en behand
- Page 12 and 13:
Stroomdiagram Diagnostiek en behand
- Page 14 and 15:
Stroomdiagram Diagnostiek en behand
- Page 16 and 17:
Stroomdiagram Revalidatietraject bi
- Page 18 and 19:
Stroomdiagram Revalidatietraject bi
- Page 20 and 21:
Samenvatting aanbevelingenAanbeveli
- Page 22 and 23:
Samenvatting aanbevelingenBehandeli
- Page 24 and 25:
Samenvatting aanbevelingenAanbeveli
- Page 26 and 27:
Samenvatting aanbevelingenAanbeveli
- Page 28 and 29:
Samenvatting aanbevelingenVia de VS
- Page 30 and 31:
Samenvatting aanbevelingenHoofdstuk
- Page 32 and 33:
Samenvatting aanbevelingenHoofdstuk
- Page 34 and 35:
Samenvatting aanbevelingenpsychosoc
- Page 36 and 37:
Samenvatting aanbevelingenHoofdstuk
- Page 38 and 39:
Samenvatting aanbevelingenAanbeveli
- Page 40 and 41:
Algemene inleidingcommunicatie tuss
- Page 42 and 43:
Algemene inleidingverder gezocht na
- Page 44 and 45:
Algemene inleidingsamenvatting van
- Page 46 and 47:
Inleiding tot de richtlijn GBSFiguu
- Page 48 and 49:
Inleiding tot de richtlijn GBSin al
- Page 50 and 51:
Inleiding tot de richtlijn GBSvan c
- Page 52 and 53:
Inleiding tot de richtlijn GBSbegel
- Page 54 and 55:
Inleiding tot de richtlijn GBSparti
- Page 56 and 57:
Inleiding tot de richtlijn GBSIn de
- Page 58 and 59:
Inleiding tot de richtlijn GBSVan D
- Page 60 and 61:
DiagnostiekVerschillende verschijni
- Page 62 and 63:
DiagnostiekBloedonderzoekHet is aan
- Page 64 and 65:
DiagnostiekEMG wordt gemaakt. Neuro
- Page 66 and 67:
DiagnostiekAanbeveling 3.1.1GBS is
- Page 68 and 69:
Medicamenteuze behandeling4. Medica
- Page 70 and 71:
Medicamenteuze behandelingDe standa
- Page 72 and 73:
Medicamenteuze behandelingHet betro
- Page 74 and 75:
Medicamenteuze behandelingVoor alle
- Page 76 and 77:
Medicamenteuze behandelingConclusie
- Page 78 and 79:
Medicamenteuze behandeling4.4 Milde
- Page 80 and 81:
Medicamenteuze behandelingDe studie
- Page 82 and 83:
Medicamenteuze behandelingOverige o
- Page 84 and 85:
Medicamenteuze behandelingAanbeveli
- Page 86 and 87:
Medicamenteuze behandelingAanbeveli
- Page 88 and 89:
Medicamenteuze behandelingVan der M
- Page 90 and 91:
Prognosehandwerk te verrichten‟ (
- Page 92 and 93:
PrognoseDe genoemde laboratoriumpar
- Page 94 and 95:
PrognoseConclusie 5.1.1Hogere leeft
- Page 96 and 97:
PrognoseEen van de belangrijkste kl
- Page 98 and 99:
PrognoseVisser LH, Schmitz PI, Meul
- Page 100 and 101:
Monitoring in de progressieve faseS
- Page 102 and 103:
StudienInclusieDesignComplicaties (
- Page 104 and 105:
Monitoring in de progressieve fase6
- Page 106 and 107:
Monitoring in de progressieve faseb
- Page 108 and 109:
Monitoring in de progressieve faseA
- Page 110 and 111:
Monitoring in de progressieve fasem
- Page 112 and 113:
Monitoring in de progressieve faseH
- Page 114 and 115:
Monitoring in de progressieve faseL
- Page 116 and 117:
Bacteriële infecties7. Bacteriële
- Page 118 and 119:
Bacteriële infectiesEén patiënt
- Page 120 and 121:
Bacteriële infectiesChiò A, Cocit
- Page 122 and 123:
IC en beademingDaarom is gezocht na
- Page 124 and 125:
IC en beademing8.2 Plaatsing trache
- Page 126 and 127:
IC en beademingAanbeveling 8.2.1Bij
- Page 128 and 129:
IC en beademinginformatie over wean
- Page 130:
IC en beademingkomt dat meestal nee
- Page 133 and 134:
CommunicatieVaak is het in het begi
- Page 135 and 136:
CommunicatieIndien mogelijk moet he
- Page 137 and 138:
Communicatieverpleegkundigen hebben
- Page 139 and 140:
Revalidatiebehandeling in het zieke
- Page 141 and 142:
Revalidatiebehandeling in het zieke
- Page 143 and 144:
Revalidatiebehandeling in het zieke
- Page 145 and 146:
Fysieke restverschijnselen11. Fysie
- Page 147 and 148:
Fysieke restverschijnselenConclusie
- Page 149 and 150:
Fysieke restverschijnselenDe Jager
- Page 151 and 152:
Vermoeidheid(zestien met GBS en vie
- Page 153 and 154:
Vermoeidheidis het van belang de tr
- Page 155 and 156:
Arbeidsre-integratieinformatie verz
- Page 157 and 158:
Arbeidsre-integratieverzekeringsart
- Page 159 and 160:
Arbeidsre-integratiesociaal functio
- Page 161 and 162:
Vaccinatie14. Vaccinatie en GBSUitg
- Page 163 and 164:
Pijn15. PijnUitgangsvragenWelke vor
- Page 165 and 166:
PijnPijnmeting bij GBSEen praktisch
- Page 167 and 168:
PijnNaast ademdepressie kunnen opio
- Page 169 and 170:
PijnDe werkgroep is van mening dat
- Page 171 and 172:
PijnFiguur 1. WHO-pijnladderAanbeve
- Page 173 and 174:
PijnMoulin DE, Hagen N, Feasby TE,
- Page 175 and 176:
Fysieke complicatiesHughes (2005) d
- Page 177 and 178:
Fysieke complicaties16.4 Heterotope
- Page 179 and 180:
Fysieke complicatiesAangezichtsverl
- Page 181 and 182:
Psychosociale aspecten17. Psychosoc
- Page 183 and 184:
Psychosociale aspectenbehoren uitvo
- Page 185 and 186:
Psychosociale aspectenHerstelfaseHe
- Page 187 and 188:
Psychosociale aspectenzich kunnen n
- Page 189 and 190:
Psychosociale aspectenEisendrath SJ
- Page 191 and 192:
Klimetrievoornamelijk gebaseerd zij
- Page 193 and 194:
KlimetrieBovendien was er een signi
- Page 195 and 196:
Klimetrievoor het meten van de part
- Page 197 and 198:
KlimetrieGebaseerd op deze aanbevel
- Page 199 and 200:
KlimetrieHolman R, Lindeboom R, Ver
- Page 201 and 202:
Training19. TrainingUitgangsvragenG
- Page 203 and 204:
TrainingForm-36 Health Questionnair
- Page 205 and 206:
Trainingde training te blijven moni
- Page 207 and 208:
TrainingAanbeveling 19.1.4In de lat
- Page 209 and 210:
Voorwaarden aan ziekenhuis en reval
- Page 211 and 212:
Voorwaarden aan ziekenhuis en reval
- Page 213 and 214:
Multidisciplinaire samenwerkingBij
- Page 215 and 216:
Multidisciplinaire samenwerkingverp
- Page 217 and 218:
Continuïteit en coördinatie van z
- Page 219 and 220:
Continuïteit en coördinatie van z
- Page 221 and 222:
FaseHerstelfase Klinischerevalidati
- Page 223 and 224:
Continuïteit en coördinatie van z
- Page 225 and 226:
224Bijlagen
- Page 227 and 228:
226Bijlagen
- Page 229 and 230:
Samenvattingskaart voor de huisarts
- Page 231 and 232:
Samenvattingskaart voor de huisarts
- Page 233 and 234:
Samenvattingskaart voor de huisarts
- Page 235 and 236:
Samenvattingskaart voor de neuroloo
- Page 237 and 238:
Samenvattingskaart voor de neuroloo
- Page 239 and 240:
Samenvattingskaart voor de neuroloo
- Page 241 and 242:
Samenvattingskaart voor de neuroloo
- Page 243 and 244:
Samenvattingskaart voor de neuroloo
- Page 245 and 246:
Samenvattingskaart voor de revalida
- Page 247 and 248:
Samenvattingskaart voor de revalida
- Page 249 and 250:
Samenvattingskaart voor de revalida
- Page 251 and 252:
Samenvattingskaart voor de revalida
- Page 253 and 254:
Samenvattingskaart voor de fysiothe
- Page 255 and 256:
Samenvattingskaart voor de fysiothe
- Page 257 and 258:
Samenvattingskaart voor de fysiothe
- Page 259 and 260:
Samenvattingskaart voor de arbeids-
- Page 261 and 262:
Samenvattingskaart voor de arbeids-
- Page 263 and 264:
Gebruikte afkortingenBijlage 6A-CID
- Page 265 and 266:
BeoordelingsschalenOnderstaande tab
- Page 267 and 268:
Beoordelingsschalen5. Overall Disab
- Page 269 and 270:
Progressieve faseBeoordelingsschale
- Page 271 and 272: Bijlagen bij conceptrichtlijn GBSBi
- Page 273 and 274: De ontwikkelde indicatoren zijn ond
- Page 275 and 276: 1. Juiste en tijdige medicamenteuze
- Page 277 and 278: zoals bloeddrukproblemen die met na
- Page 279 and 280: RekenregelEen aantal variabelen die
- Page 281 and 282: 2. Regelmatige pijnmeting bij pati
- Page 283 and 284: standaardcontroles van de verplegin
- Page 285 and 286: informatiesysteem. Begin voor het b
- Page 287 and 288: De (kinder)revalidatiearts is belan
- Page 289 and 290: 4. Vastlegging belangrijkste factor
- Page 291 and 292: een hoofdbehandelaar te benoemen. D
- Page 293 and 294: Bijlage 10Evidence tabellenBewijskl
- Page 295 and 296: Greenwood, 1984 B Gerandomiseerdetr
- Page 297 and 298: Visser, 1999 C Prospectieve studieb
- Page 299 and 300: Cheng,2000Bpatiënten debelangrijks
- Page 301 and 302: kinderen met GBSverwezen naar FTen
- Page 303 and 304: Bewijsklassetabel behorend bij hoof
- Page 305 and 306: Auteur,jaartalBewijsklassetabel beh
- Page 307 and 308: Bewijsklassetabellen bij Multidisci
- Page 309 and 310: 2003 studie in eendatabase met 114p
- Page 311 and 312: Bernsen,1999Bernsen,2005De Jager,19
- Page 313 and 314: Vedeler,1997Korinthenberg, 2007Koep
- Page 315 and 316: Garssen,2006Garssen,2004Ruhland,199
- Page 317 and 318: Bewijsklassetabel behorend bij GBS
- Page 319 and 320: Lennon,1993Dornonvillede la Cour,20
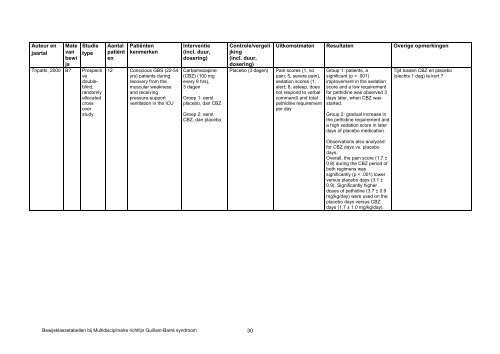
- Page 321: Auteur enjaartalPandey, 2005 BMatev
- Page 325 and 326: patiënten ernstigepijnklachten waa
- Page 327 and 328: van de variatie inhandicap-scores.M
- Page 329 and 330: Graham,2006Merkies,2000Cross-sectio
- Page 331: Bewijsklassetabel behorend bij hoof