Third Day Poster Session, 17 June 2010 - NanoTR-VI
Third Day Poster Session, 17 June 2010 - NanoTR-VI
Third Day Poster Session, 17 June 2010 - NanoTR-VI
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Poster</strong> <strong>Session</strong>, Thursday, <strong>June</strong> <strong>17</strong><br />
Theme F686 - N1123<br />
Synthesis of Noncarbon Nanotubes/ conductive Polymer nanocomposite in free solvent media<br />
G.R. Kiani 1* and A. Rostami 2<br />
1 Department of Applied Chemistry, Faculty of Science, University of Maragheh, Maragheh, Iran<br />
2 School of engineering, emerging technologies, University of Tabriz, Tabriz, Iran<br />
Abstract- Conducting polymer/ halloysite nanotubes composite were obtained by a mechanochemical reaction in the<br />
solid state by milling system. The halloysite nanotubes(HNT) were coated with 2, 5- dithienyl pyrrole(SNS) via the<br />
in situ chemical oxidation polymerization. The characterization by transmission electron microscopy showed that<br />
the nanotubes were completely covered with conducting polymer. The nanocomposite was characterized using<br />
FTIR, X-ray diffraction, TGA and transmission electron microscopy. The conductivity of the nanocomposite was<br />
found to be 0.0066 (cm) -1 . (HNT) provide a new avenue for the preparation of nanocomposites.<br />
Conducting polymer nanotubes have recently<br />
become the object of numerous investigations<br />
because of their great potential in device<br />
applications, such as transistors [1], sensors [2],<br />
actuators and batteries [3], and so on. Holloysite has<br />
a wide variety of biological and non-biological<br />
applications. It has been used for storing molecular<br />
hydrogen [4], for catalyst conversions and<br />
processing of hydrocarbons [5] and for removing<br />
environmental hazardous species [6].<br />
HNT and SNS in the presence of ammonium<br />
presulphate were placed in the ball milling apparatus<br />
and the mixture was milled for 30 minute at room<br />
temperature (25 Hz). The black powder of HNT-SNS<br />
nanocomposite was washed with water, and ethanol,<br />
then the dried in vacuum.<br />
TEM images of halloysite nanotubes (HNT) and<br />
HNT-SNS show that the halloysite nanotubes were<br />
coated with 2, 5- dithienyl pyrrole(SNS) via the in<br />
situ chemical oxidation polymerization. Also it is<br />
obvious that the poly SNS layer with the thickness of<br />
about 40 nm was only coated onto the outer surfaces<br />
of the HNTs.<br />
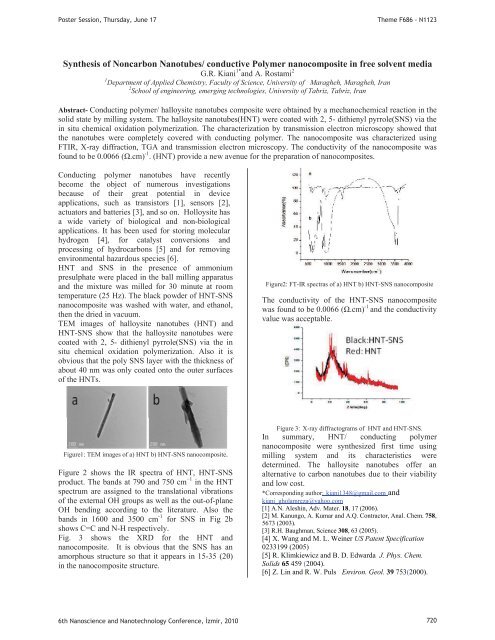
Figure2: FT-IR spectras of a) HNT b) HNT-SNS nanocomposite<br />
The conductivity of the HNT-SNS nanocomposite<br />
was found to be 0.0066 (cm) -1 and the conductivity<br />
value was acceptable.<br />
Figure1: TEM images of a) HNT b) HNT-SNS nanocomposite.<br />
Figure 2 shows the IR spectra of HNT, HNT-SNS<br />
product. The bands at 790 and 750 cm 1 in the HNT<br />
spectrum are assigned to the translational vibrations<br />
of the external OH groups as well as the out-of-plane<br />
OH bending according to the literature. Also the<br />
bands in 1600 and 3500 cm -1 for SNS in Fig 2b<br />
shows C=C and N-H respectively.<br />
Fig. 3 shows the XRD for the HNT and<br />
nanocomposite. It is obvious that the SNS has an<br />
amorphous structure so that it appears in 15-35 (2)<br />
in the nanocomposite structure.<br />
Figure 3: X-ray diffractograms of HNT and HNT-SNS.<br />
In summary, HNT/ conducting polymer<br />
nanocomposite were synthesized first time using<br />
milling system and its characteristics were<br />
determined. The halloysite nanotubes offer an<br />
alternative to carbon nanotubes due to their viability<br />
and low cost.<br />
*Corresponding author: kiani1348@gmail.com and<br />
kiani_gholamreza@yahoo.com<br />
[1] A.N. Aleshin, Adv. Mater. 18, <strong>17</strong> (2006).<br />
[2] M. Kanungo, A. Kumar and A.Q. Contractor, Anal. Chem. 758,<br />
5673 (2003).<br />
[3] R.H. Baughman, Science 308, 63 (2005).<br />
[4] X. Wang and M. L. Weiner US Patent Specification<br />
0233199 (2005)<br />
[5] R. Klimkiewicz and B. D. Edwarda J. Phys. Chem.<br />
Solids 65 459 (2004).<br />
[6] Z. Lin and R. W. Puls Environ. Geol. 39 753(2000).<br />
6th Nanoscience and Nanotechnology Conference, zmir, <strong>2010</strong> 720