Third Day Poster Session, 17 June 2010 - NanoTR-VI
Third Day Poster Session, 17 June 2010 - NanoTR-VI
Third Day Poster Session, 17 June 2010 - NanoTR-VI
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Poster</strong> <strong>Session</strong>, Thursday, <strong>June</strong> <strong>17</strong><br />
Theme F686 - N1123<br />
Direct measurement of humidity adsorption kinetics of Calix[4]arene derivative using QCM technique<br />
Omer Mermer 1* , Salih Okur 2 , Fevzi Sümer 1 , Mahmut Ku 3 , eref Ertul 4 , Mevlüt Bayrakç 4 , Mustafa Ylmaz 4<br />
1 Ege University, Department of Electrical and Electronics Engineering Bornova/Izmir/TURKEY<br />
2 Izmir Institute of Technology, Department of Physics Urla/Izmir/TURKEY<br />
3 Selçuk University, Department of Chemical Engineering, Selçuklu/Konya/TURKEY<br />
4 Selçuk University, Department of Chemistry, Selçuklu/Konya/TURKEY<br />
Abstract— This study focuses on the optimization and characterization of calix[4]arene derivative based sensor film coated on<br />
a quartz substrate by drop casting method for use in the detection of humidity. The humidity adsorption and desorption kinetics<br />
calix[4]arene were investigated by Quartz Crystal Microbalance (QCM) technique. The Langmuir model was used to<br />
determine the kinetic parameters such as adsorption, desorption rates and Gibbs free energy between relative humidity between<br />
11% and 97%. Our reproducible experimental results show that calix[4]arene films have a great potential for humidity sensing<br />
applications at room temperature operations.<br />
Monitoring and control of humidity is essential for<br />
industrial progress of the world such as petroleum industry,<br />
medical equipments, food industry and the manufacturer of<br />
moisture sensitive products. Furthermore clean rooms,<br />
greenhouses, research and developments labs are all<br />
environments that are highly effected by moisture levels and<br />
require constant monitoring [1–2].<br />
Quartz crystal microbalances (QCMs) have been widely<br />
used in recently as promising gas sensor applications owing to<br />
their high sensitivity and ease of measurement, since the<br />
measured frequency shift is directly proportional to the mass<br />
change on a quartz crystal [1–2].<br />
Thin films of calix[4]arene derivatives have been widely<br />
used in chemical sensors. Due to their zeolite-like capacity<br />
and selectivity, calix[4]arene became promising materials for<br />
sensor applications. The functional groups at the upper and<br />
lower rims determine their selectivity in host-guest<br />
interactions and physical properties [3]. Calix[4]arene<br />
derivatives have been used in recent times as gas sensors<br />
applications [4,5].<br />
The frequency response curve QCM-based sensor to<br />
cyclic humidity change is depicted in Figure 2 for different<br />
RH levels. The frequency shift of QCM decreases sharply<br />
with increasing humidity concentrations while there is no<br />
change in that of the empty QCM (adsorption process). On the<br />
other hand, during the desorption process, the humidity level<br />
is turned back to the initial value, as a result, QCM recovers<br />
back to its initial resonance frequency value.<br />
Langmuir adsorption isotherm model is frequently<br />
used to describe adsorption and desorption kinetics of gas<br />
vapor molecules onto organic or inorganic films [6-7].<br />
According to this model, the rate of surface reaction for<br />
forming a monolayer on the surface is related to fractional<br />
coverage (), the humidity concentration, and the rate<br />
constants for the adsorption (k a ) and desorption (k d ) processes.<br />
Figure 3 shows the experimental data and the fitting curve for<br />
first adsorption cycle. k a and k d fitting parameters determined<br />
from the fit using Langmuir equation to the experimental data<br />
are given by 62.49 M -1 s -1 and 0.0005 s -1 , respectively [8].<br />
10<br />
8<br />
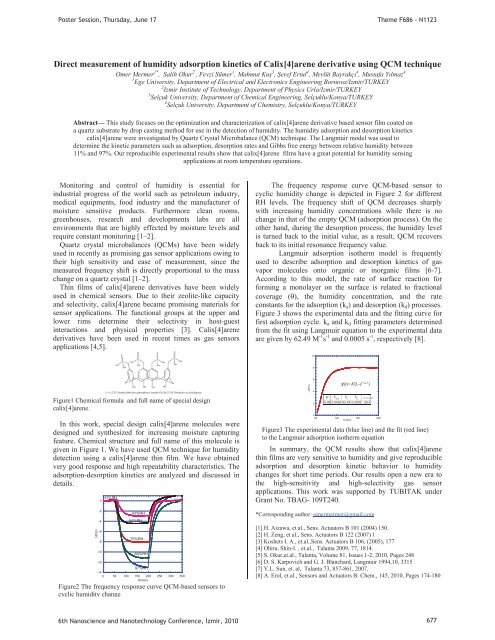
Figure1 Chemical formula and full name of special design<br />
calix[4]arene.<br />
In this work, special design calix[4]arene molecules were<br />
designed and synthesized for increasing moisture capturing<br />
feature. Chemical structure and full name of this molecule is<br />
given in Figure 1. We have used QCM technique for humidity<br />
detection using a calix[4]arene thin film. We have obtained<br />
very good response and high repeatability characteristics. The<br />
adsorption-desorption kinetics are analyzed and discussed in<br />
details.<br />
f(Hz)<br />
0<br />
-2<br />
-4<br />
-6<br />
-8<br />
-10<br />
-12<br />
-14<br />
11%RH<br />
43%RH<br />
54%RH<br />
75%RH<br />
84%RH<br />
97%RH<br />
0 50 100 150 200 250 300 350<br />
time(s)<br />
Figure2 The frequency response curve QCM-based sensors to<br />
cyclic humidity change<br />
-f(Hz)<br />
6<br />
4<br />
2<br />
K'<br />
8.08<br />
k obs<br />
0.0455<br />
f<br />
( t)<br />
K(1<br />
e<br />
k a<br />
62.49<br />
( kobst)<br />
0<br />
50 100 150 200<br />
time(s)<br />
k d<br />
0.0005<br />
)<br />
G (kJ/mol)<br />
Figure3 The experimental data (blue line) and the fit (red line)<br />
to the Langmuir adsorption isotherm equation<br />
In summary, the QCM results show that calix[4]arene<br />
thin films are very sensitive to humidity and give reproducible<br />
adsorption and desorption kinetic behavior to humidity<br />
changes for short time periods. Our results open a new era to<br />
the high-sensitivity and high-selectivity gas sensor<br />
applications. This work was supported by TUBITAK under<br />
Grant No. TBAG- 109T240.<br />
-29.3<br />
*Corresponding author: omermermer@gmail.com<br />
[1] H. Aizawa, et al., Sens. Actuators B 101 (2004) 150.<br />
[2] H. Zeng, et al., Sens. Actuators B 122 (2007) 1<br />
[3] Koshets I. A., et.al.,Sens. Actuators B 106, (2005), <strong>17</strong>7<br />
[4] Ohira, Shin-I. , et.al., Talanta 2009, 77, 1814.<br />
[5] S. Okur,et.al., Talanta, Volume 81, Issues 1-2, <strong>2010</strong>, Pages 248<br />
[6] D. S. Karpovich and G. J. Blanchard, Langmuir 1994,10, 3315<br />
[7] Y.L. Sun, et. al, Talanta 73, 857-861, 2007.<br />
[8] A. Erol, et.al., Sensors and Actuators B: Chem., 145, <strong>2010</strong>, Pages <strong>17</strong>4-180<br />
6th Nanoscience and Nanotechnology Conference, zmir, <strong>2010</strong> 677