Third Day Poster Session, 17 June 2010 - NanoTR-VI
Third Day Poster Session, 17 June 2010 - NanoTR-VI
Third Day Poster Session, 17 June 2010 - NanoTR-VI
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Poster</strong> <strong>Session</strong>, Thursday, <strong>June</strong> <strong>17</strong><br />
Theme F686 - N1123<br />
Hydrogen Chemisorption on Metal Loaded Carbon Nanotubes<br />
Gülah Ozan 1* , Saadet Kayıran Beyaz 2 , Nalan Tekin 2 , M. Efkan Kibar 3<br />
1 Gebze Institute of Technology, Department of Chemistry, 41400 Gebze / Kocaeli-TURKEY.<br />
2 Kocaeli University, Faculty of Science and Arts, Department of Chemistry, 41380 Izmit / Kocaeli- TURKEY.<br />
3 Kocaeli University, Faculty of Engineering, Department of Chemical Engineering, 41380 Izmit / Kocaeli- TURKEY.<br />
Abstract— Metal doped or metal decorated carbon nanotubes (CNTs) are the most capable materials for hydrogen storage by<br />
chemisorption. Using CNTs for hydrogen storage has been one of the hottest topics in science and technology. We have<br />
developed nickel doped materials by wet chemistry method and measured the hydrogen absorption capacity of these materials.<br />
The comparison of absorption capacities between materials is discussed in the light of characterization results.<br />
Hydrogen storage is an essential prerequisite for the<br />
widespread deployment of fuel cells, particularly in transport.<br />
The US Department of Energy (DOE) has announced a 6.0<br />
wt% target for hydrogen storage on-board automobiles [1, 2].<br />
Sorption in solids is one of the way for increasing hydrogen<br />
storage. Nanostructured carbons are being investigated as<br />
potential hydrogen adsorbents since they are cheap, light and<br />
porous materials. CNTs can easily and dependably accept and<br />
release substantial quantities of hydrogen by physisorption and<br />
chemisorption mechanisms [3]. Many works are devoted to<br />
measure and calculate hydrogen adsorption capacity of carbon<br />
nanomaterials. According to the works done by Monte Carlo<br />
simulations, it is observed that hydrogen storage is less than<br />
1% at room temperature when it is absorbed with various<br />
carbon materials such as SWCNTs, MWCNTs, and GNFs.<br />
These works show that the physisorption is not sufficent for<br />
store the quantity of hydrogen given in DOE target [4-6]. For<br />
this reason the researchers have tried to create more attractive<br />
nanocarbons surface for creating strong interaction with<br />
hydrogen. The chemisorption mechanism could provide the<br />
hydrogen storage capacity which can fulfill the technological<br />
necessity [3]. The aim of this work is to study the hydrogen<br />
chemisorption on Ni doped materials.<br />
In this study, three different Ni doped materials were<br />
prepared using active carbon (AC), Al 2 O 3 and multi walled<br />
carbon nanotubes (MWCNTs). MWCNTs were synthesized<br />
by Chemical Vapor Deposition (CVD) method using acetylene<br />
as a carbon source and nickel as catalyst. MWCNTs were<br />
purified by thermal oxidation in air at temperature at 350 ºC<br />
for 2h in order to remove amorphous carbon and were treated<br />
with 3M HCl solution in order to remove metal catalyst.<br />
Carboxylic acid functionalized MWCNTs (f-MWCNTs) have<br />
been obtained by treatment of concentrated H 2 SO 4 /HNO 3 (v:v,<br />
3:1). 15 % Ni doped f-MWNTs, Al 2 O 3 and AC were prepared<br />
from Ni(NO 3 ) 2 .6H 2 O by wet chemistry method. Formerly, f-<br />
MWNT, Al 2 O 3 and A.C. was ultrasonically dispersed in<br />
ethanol for 3h and Ni(NO 3 ) 2 .6H 2 O solution was added and<br />
ultrasonicated in order to obtain homogenous metal dispersion<br />
on these materials. Ni doped materials were centrifuged and<br />
dried at 60 ºC for 4 days in vacuum oven. The synthesized<br />
MWCNTs, f-MWCNTs were characterized by TEM, SEM,<br />
FT-IR, EDX, Raman, and BET techniques. The specific<br />
surface area of the 15% Ni doped materials are 222 m2/g for f-<br />
MWCNTs, 114m2/g for Al 2 O 3 and 362m2/g for AC. The<br />
hydrogen chemisorptions capacity of obtained Ni doped<br />
materials was measured using Micromeritics, ASAP 2020,<br />
Surface Area and Porosity Analyzer. Figure clearly shows that<br />
MWCNTs were successfully synthesized and doped with Ni<br />
metal atoms.<br />
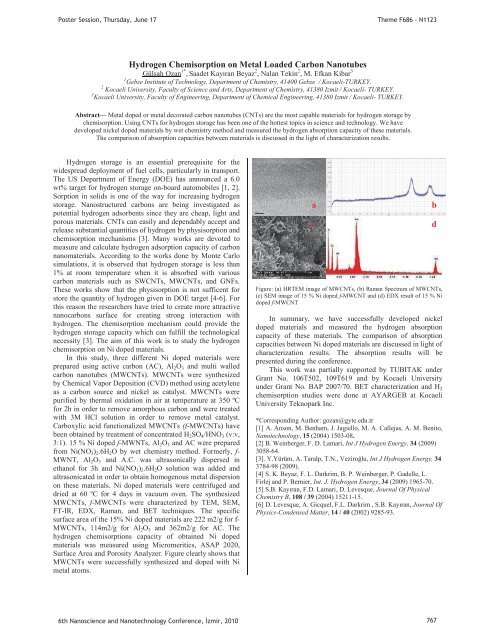
a<br />
c<br />
Figure: (a) HRTEM image of MWCNTs, (b) Raman Spectrum of MWCNTs,<br />
(c) SEM image of 15 % Ni doped f-MWCNT and (d) EDX result of 15 % Ni<br />
doped f-MWCNT<br />
In summary, we have successfully developed nickel<br />
doped materials and measured the hydrogen absorption<br />
capacity of these materials. The comparison of absorption<br />
capacities between Ni doped materials are discussed in light of<br />
characterization results. The absorption results will be<br />
presented during the conference.<br />
This work was partially supported by TUBITAK under<br />
Grant No. 106T502, 109T619 and by Kocaeli University<br />
under Grant No. BAP 2007/70. BET characterization and H 2<br />
chemisorption studies were done at AYARGEB at Kocaeli<br />
University Teknopark Inc.<br />
*Corresponding Author: gozan@gyte.edu.tr<br />
[1] A. Anson, M. Benham, J. Jagiello, M. A. Callejas, A. M. Benito,<br />
Nanotechnology, 15 (2004) 1503-08.<br />
[2] B. Weinberger, F. D. Lamari, Int J Hydrogen Energy, 34 (2009)<br />
3058-64.<br />
[3]. Y.Yürüm, A. Taralp, T.N., Vezirolu, Int J Hydrogen Energy, 34<br />
3784-98 (2009).<br />
[4] S. K. Beyaz, F. L. Darkrim, B. P. Weinberger, P. Gadelle, L.<br />
Firlej and P. Bernier, Int. J. Hydrogen Energy, 34 (2009) 1965-70.<br />
[5] S.B. Kayıran, F.D. Lamari, D. Levesque, Journal Of Physical<br />
Chemistry B, 108 / 39 (2004) 15211-15.<br />
[6] D. Levesque, A. Gicquel, F.L. Darkrim , S.B. Kayıran, Journal Of<br />
Physics-Condensed Matter, 14 / 40 (2002) 9285-93.<br />
b<br />
d<br />
6th Nanoscience and Nanotechnology Conference, zmir, <strong>2010</strong> 767