Photonic crystals in biology
Photonic crystals in biology
Photonic crystals in biology
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Poster Session, Tuesday, June 15<br />
Theme A1 - B702<br />
Multiwalled carbon nanotube modified with 5-Br-PADAP for stripp<strong>in</strong>g voltammetric<br />
determ<strong>in</strong>ation of Pb(II)<br />
Ashraf Salmanipour, 1 Mohammad Ali Taher 1 and Alireza Mohadesi 2*<br />
1<br />
Department of Chemistry, Shahid Bahonar University of Kerman, P.O. Box 76175-133, Kerman, Iran<br />
2<br />
Department of Chemistry, Payame Noor University (PNU), P.O. Box 76175-559, Kerman, Iran<br />
Abstract— In this work, we have demonstrated that glassy carbon electrode (GCE) modified with multiwalled carbon<br />
nanotube (MWNT) functionalized with 2-(5-bromo-2-pyridylazo)-5-diethylam<strong>in</strong>ophenol (5-Br-PADAP) as a ligand can be<br />
used for the stripp<strong>in</strong>g voltammetry of lead(II). The ability of the 5-Br-PADAP to extract Pb(II) <strong>in</strong>to the electrode surface leads<br />
to an electrochemical sensor that is precise and accurate for determ<strong>in</strong>ation of lead(II) <strong>in</strong> water samples.<br />
Adsorptive stripp<strong>in</strong>g voltammetry generally applies an<br />
accumulation step prior to the voltammetric scan <strong>in</strong> order to<br />
develop analysis methods with higher levels of sensitivity. In<br />
this step, analyte (metal ion) is adsorbed from sample solution<br />
to electrode surface based on complexation between metal ion<br />
and ligand immobilized on the electrode surface. In this sense,<br />
ow<strong>in</strong>g to the strong sorption properties of carbon nanotubes<br />
and their advantages <strong>in</strong> electrochemical measurements, this<br />
nanostructured material has allowed some novel methods of<br />
stripp<strong>in</strong>g analysis to be developed [1-4].<br />
In this work, the MWNT functionalized with a complex<br />
reagent (5-Br-PADAP) was prepared and coated on a GCE.<br />
This modified electrode was used successfully for anodic<br />
stripp<strong>in</strong>g determ<strong>in</strong>ation of lead(II) <strong>in</strong> some real samples.<br />
Before use, the MWNTs were purified and pretreated to<br />
remove graphitic nanoparticles, amorphous carbon, and<br />
catalyst impurities, and then functionalized with carboxylic<br />
acid groups accord<strong>in</strong>g to the literature [5]. After these<br />
procedures, the carboxylic acid groups were <strong>in</strong>troduced onto<br />
the cross sections of the MWNTs. For preparation of 5-Br-<br />
PADAP-functionalized MWNT, the 5-Br-PADAP dissolved<br />
<strong>in</strong> ethanol was added to 1 g of pretreated MWNT, step by step<br />
and the solution was stirred for 24 hour till the solution<br />
became colorless. Then the modified MWNTs were filtered<br />
and washed with doubly distilled water and got dried at room<br />
temperature. Then ultrasonication agitation for 20 m<strong>in</strong> was<br />
applied to disperse 0.5 mg 5-Br-PADAP/MWNT <strong>in</strong>to 1 ml of<br />
double distilled water to give 0.5 mg/ml suspension. Before<br />
coat<strong>in</strong>g, the GCE was polished with a nano-Al 2 O 3 powder on a<br />
polish<strong>in</strong>g pad r<strong>in</strong>sed thoroughly with doubly distilled water,<br />
then sonicated <strong>in</strong> aceton for 2 m<strong>in</strong>. F<strong>in</strong>ally, the GCE was<br />
coated with 10 μL of 0.5 mg/ml 5-Br-PADAP/MWNT<br />
suspension and the water allowed evaporat<strong>in</strong>g at room<br />
temperature <strong>in</strong> the air. The bare electrode was prepared by the<br />
same procedure just with unfunctionalized MWNTs.<br />
For differential pulse anodic (DPA) or cyclic stripp<strong>in</strong>g<br />
voltammetric experiments, the 5-Br-PADAP/MWNT/GCE<br />
was immersed <strong>in</strong> a 25 ml of sample solution (0.1 M acetate<br />
buffer pH 5.58) conta<strong>in</strong><strong>in</strong>g a known amount of Pb(II) and the<br />
solution was stirred for 15 m<strong>in</strong>. This was followed by medium<br />
exchange to a 0.1 M stripp<strong>in</strong>g solution (0.1 M KNO 3 , 0.1 M<br />
acetate buffer pH 4.23), where the DPA or cyclic<br />
voltammograms were recorded from -1.0 to -0.2 V.<br />
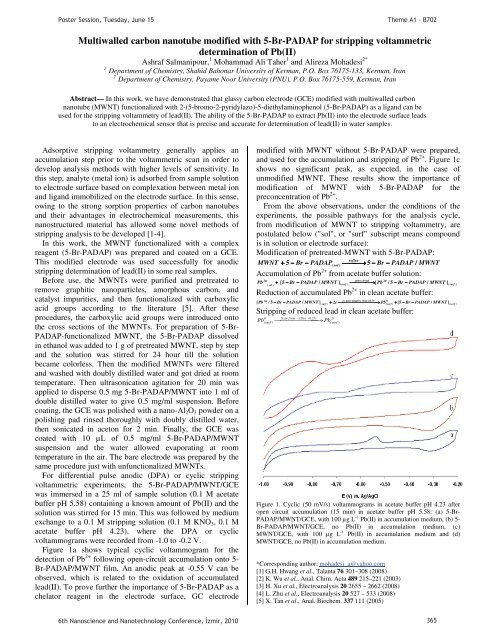
Figure 1a shows typical cyclic voltammogram for the<br />
detection of Pb 2+ follow<strong>in</strong>g open-circuit accumulation onto 5-<br />
Br-PADAP/MWNT film. An anodic peak at -0.55 V can be<br />
observed, which is related to the oxidation of accumulated<br />
lead(II). To prove further the importance of 5-Br-PADAP as a<br />
chelator reagent <strong>in</strong> the electrode surface, GC electrode<br />
modified with MWNT without 5-Br-PADAP were prepared,<br />
and used for the accumulation and stripp<strong>in</strong>g of Pb 2+ . Figure 1c<br />
shows no significant peak, as expected, <strong>in</strong> the case of<br />
unmodified MWNT. These results show the importance of<br />
modification of MWNT with 5-Br-PADAP for the<br />
preconcentration of Pb 2+ .<br />
From the above observations, under the conditions of the<br />
experiments, the possible pathways for the analysis cycle,<br />
from modification of MWNT to stripp<strong>in</strong>g voltammetry, are<br />
postulated below ("sol", or "surf" subscript means compound<br />
is <strong>in</strong> solution or electrode surface):<br />
Modification of pretreated-MWNT with 5-Br-PADAP:<br />
reflux<br />
MWNT + 5 − Br − PADAP( sol )<br />
⎯⎯⎯<br />
→ 5 − Br − PADAP / MWNT<br />
Accumulation of Pb 2+ from acetate buffer solution:<br />
Pb<br />
2+<br />
(<br />
sol<br />
)<br />
+<br />
open circuit 2+<br />
[ 5 − Br − PADAP / MWNT ](<br />
surf )<br />
⎯⎯ ⎯⎯→<br />
[ Pb / 5 − Br − PADAP / MWNT ](<br />
surf<br />
Reduction of accumulated Pb 2+ <strong>in</strong> clean acetate buffer:<br />
2+<br />
<strong>in</strong> more negative than −0.7V<br />
0<br />
[ Pb / 5 − Br − PADAP / MWNT]<br />
( surf )<br />
+ 2e<br />
⎯⎯⎯⎯⎯⎯⎯⎯→<br />
Pb(<br />
surf )<br />
+ [5 − Br − PADAP / MWNT]<br />
( surf<br />
Stripp<strong>in</strong>g of reduced lead <strong>in</strong> clean acetate buffer:<br />
Pb<br />
⎯⎯⎯⎯⎯⎯⎯→Pb<br />
0 Scan from −1.0<br />
to −0.2V<br />
2+<br />
( surf )<br />
( surf )<br />
Figure 1. Cyclic (50 mV/s) voltammograms <strong>in</strong> acetate buffer pH 4.23 after<br />
open circuit accumulation (15 m<strong>in</strong>) <strong>in</strong> acetate buffer pH 5.58: (a) 5-Br-<br />
PADAP/MWNT/GCE, with 100 μg L -1 Pb(II) <strong>in</strong> accumulation medium, (b) 5-<br />
Br-PADAP/MWNT/GCE, no Pb(II) <strong>in</strong> accumulation medium, (c)<br />
MWNT/GCE, with 100 μg L -1 Pb(II) <strong>in</strong> accumulation medium and (d)<br />
MWNT/GCE, no Pb(II) <strong>in</strong> accumulation medium.<br />
*Correspond<strong>in</strong>g author: mohadesi_a@yahoo.com<br />
[1] G.H. Hwang et al., Talanta 76 301–308 (2008)<br />
[2] K. Wu et al., Anal. Chim. Acta 489 215–221 (2003)<br />
[3] H. Xu et al., Electroanalysis 20 2655 – 2662 (2008)<br />
[4] L. Zhu et al., Electroanalysis 20 527 – 533 (2008)<br />
[5] X. Tan et al., Anal. Biochem. 337 111 (2005)<br />
)<br />
)<br />
6th Nanoscience and Nanotechnology Conference, zmir, 2010 365