Photonic crystals in biology
Photonic crystals in biology
Photonic crystals in biology
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Poster Session, Tuesday, June 15<br />
Theme A1 - B702<br />
Investigation of nucleation and growth mechanism dur<strong>in</strong>g formation of poly(azure A)<br />
Emrah Kalyoncu, Kader Dacı, brahim Hakkı Kaplan, Ezgi Topçu, Murat Alanyalıolu*<br />
Department of Chemistry, Sciences Faculty, Atatürk University, Erzurum 25240, Turkey<br />
Abstract—Nucleation and growth mechanism dur<strong>in</strong>g formation of poly(AA) films on gold substrates was <strong>in</strong>testigated. Repeated potential<br />
cycl<strong>in</strong>g by us<strong>in</strong>g cyclic voltammetry and potential controlled electrolysis techniques have been performed to synthesize poly(AA) th<strong>in</strong> films on<br />
gold work<strong>in</strong>g electrodes <strong>in</strong> the solution conta<strong>in</strong><strong>in</strong>g 0.1 mM AA and 0.1 M phosphate solution (pH:6.2). Chronoamperometry, STM (Scann<strong>in</strong>g<br />
Tunnel<strong>in</strong>g Microscopy), AFM (Atomic Force Microscopy), and UV-vis. absorption spectroscopy techniques were applied for the characterization<br />
of prepared polymeric films.<br />
Dye polymer films show excellent catalytic and<br />
photoelectrochemical properties and have been applied for<br />
batteries and electrodes, electrochromic devices, light emitt<strong>in</strong>g<br />
diodes, and immobilizitaion of enzymes [1,2]. Dye polymer<br />
films must have a well-ordered surface to be used <strong>in</strong> these<br />
technological applications. Electropolymerization is one of the<br />
simple and useful method to obta<strong>in</strong> dye polymer films. In the<br />
electropolymerization process, deposition of polymeric dye<br />
film on the electrode surface is achieved by constant or cycled<br />
potential oxidation of a dye-conta<strong>in</strong><strong>in</strong>g solution. Azure A is a<br />
derivative of phenothiaz<strong>in</strong>e dye material (Figure 1).<br />
for progressive case. Growth of nuclei is slow for<br />
<strong>in</strong>stantaneous nucleation and fast for progressive nucleation<br />
[7,8]. Accord<strong>in</strong>g to Li and Albery [9], two possible<br />
mechanisms are possible for polymer films: Progressive twodimensional<br />
layer-by-layer nucleation and <strong>in</strong>stantaneous threedimensional<br />
nucleation and growth. In this study,<br />
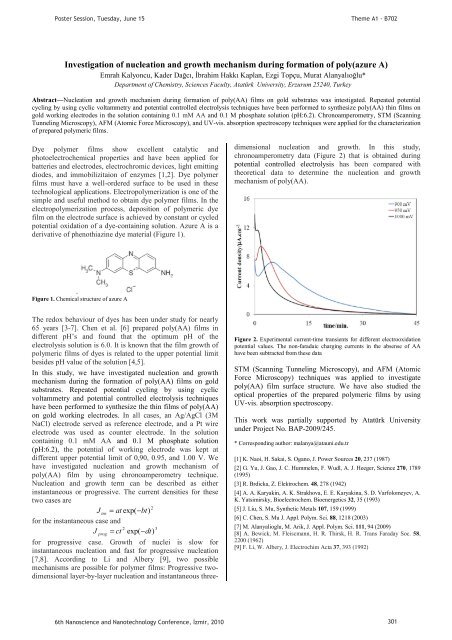
chronoamperometry data (Figure 2) that is obta<strong>in</strong>ed dur<strong>in</strong>g<br />
potential controlled electrolysis has been compared with<br />
theoretical data to determ<strong>in</strong>e the nucleation and growth<br />
mechanism of poly(AA).<br />
.<br />
Figure 1. Chemical structure of azure A<br />
The redox behaviour of dyes has been under study for nearly<br />
65 years [3-7]. Chen et al. [6] prepared poly(AA) films <strong>in</strong><br />
different pH’s and found that the optimum pH of the<br />
electrolysis solution is 6.0. It is known that the film growth of<br />
polymeric films of dyes is related to the upper potential limit<br />
besides pH value of the solution [4,5].<br />
In this study, we have <strong>in</strong>vestigated nucleation and growth<br />
mechanism dur<strong>in</strong>g the formation of poly(AA) films on gold<br />
substrates. Repeated potential cycl<strong>in</strong>g by us<strong>in</strong>g cyclic<br />
voltammetry and potential controlled electrolysis techniques<br />
have been performed to synthesize the th<strong>in</strong> films of poly(AA)<br />
on gold work<strong>in</strong>g electrodes. In all cases, an Ag/AgCl (3M<br />
NaCl) electrode served as reference electrode, and a Pt wire<br />
electrode was used as counter electrode. In the solution<br />
conta<strong>in</strong><strong>in</strong>g 0.1 mM AA and 0.1 M phosphate solution<br />
(pH:6.2), the potential of work<strong>in</strong>g electrode was kept at<br />
different upper potential limit of 0,90, 0.95, and 1.00 V. We<br />
have <strong>in</strong>vestigated nucleation and growth mechanism of<br />
poly(AA) film by us<strong>in</strong>g chronoamperometry technique.<br />
Nucleation and growth term can be described as either<br />
<strong>in</strong>stantaneous or progressive. The current densities for these<br />
two cases are<br />
2<br />
J <strong>in</strong>s<br />
= at exp( −bt)<br />
for the <strong>in</strong>stantaneous case and<br />
2<br />
3<br />
= ct exp( −dt)<br />
J prog<br />
Figure 2. Experimental current-time transients for different electrooxidation<br />
potential values. The non-faradaic charg<strong>in</strong>g currents <strong>in</strong> the absense of AA<br />
have been subtracted from these data<br />
STM (Scann<strong>in</strong>g Tunnel<strong>in</strong>g Microscopy), and AFM (Atomic<br />
Force Microscopy) techniques was applied to <strong>in</strong>vestigate<br />
poly(AA) film surface structure. We have also studied the<br />
optical properties of the prepared polymeric films by us<strong>in</strong>g<br />
UV-vis. absorption spectroscopy.<br />
This work was partially supported by Atatürk University<br />
under Project No. BAP-2009/245.<br />
* Correspond<strong>in</strong>g author: malanya@atauni.edu.tr<br />
[1] K. Naoi, H. Sakai, S. Ogano, J. Power Sources 20, 237 (1987)<br />
[2] G. Yu, J. Gao, J. C. Hummelen, F. Wudl, A. J. Heeger, Science 270, 1789<br />
(1995)<br />
[3] R. Brdicka, Z. Elektrochem. 48, 278 (1942)<br />
[4] A. A. Karyak<strong>in</strong>, A. K. Strakhova, E. E. Karyak<strong>in</strong>a, S. D. Varfolomeyev, A.<br />
K. Yatsimirsky, Bioelectrochem. Bioenergetics 32, 35 (1993)<br />
[5] J. Liu, S. Mu, Synthetic Metals 107, 159 (1999)<br />
[6] C. Chen, S. Mu J. Appl. Polym. Sci. 88, 1218 (2003)<br />
[7] M. Alanyalioglu, M. Arik, J. Appl. Polym. Sci. 111, 94 (2009)<br />
[8] A. Bewick, M. Fleiscmann, H. R. Thirsk, H. R. Trans Faraday Soc. 58,<br />
2200 (1962)<br />
[9] F. Li, W. Albery, J. Electrochim Acta 37, 393 (1992)<br />
6th Nanoscience and Nanotechnology Conference, zmir, 2010 301