Photonic crystals in biology
Photonic crystals in biology
Photonic crystals in biology
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Poster Session, Tuesday, June 15<br />
Theme A1 - B702<br />
Preparation and characterization of silver nanoparticles by an eco-friendly approach<br />
Reda El-Sh ishtawy 1 * Abdullah Asiri 1 and Maha Al-Otaibi 1<br />
Department of Chemistry, Faculty of Science, K<strong>in</strong>g Abdul-Aziz University, Jeddah 21589, PO Box 80203, Saudi Arabia<br />
1<br />
Abstract-This work reports on an environmentally benign method for the preparation of AgNPs <strong>in</strong> aqueous solution us<strong>in</strong>g glucose as the<br />
reduc<strong>in</strong>g agent <strong>in</strong> water/ micelles system, <strong>in</strong> which cetyltrimethylammonium bromide (CTAB) was used as capp<strong>in</strong>g agent (stabilizer).<br />
Spectrophotometric method was used to monitor the formation of AgNPs under different conditions such as a) concentration of sodium<br />
hydroxide, b) concentration of glucose, c) concentration of silver nitrate d) concentration of CTAB, and e) reaction time were used so as to f<strong>in</strong>d<br />
out the optimum conditions for the preparation of AgNPs.<br />
Silver nanoparticles (AgNPs) have received much more<br />
attention <strong>in</strong> recent years due to their unique physical, optical,<br />
electrical, magnetic, and chemical properties and their<br />
potential application <strong>in</strong> molecular electronic field [1].<br />
Therefore, extensive research has gone <strong>in</strong>to develop<strong>in</strong>g<br />
suitable preparation techniques to form AgNPs. These <strong>in</strong>clude<br />
chemical reduction, microorganisms <strong>in</strong>duced reduction as well<br />
as photochemical and high energy radiation <strong>in</strong>duced reduction<br />
of silver salt solution. Among these methods, chemical<br />
reduction is the most extensively <strong>in</strong>vestigated method [2-6].<br />
Figure 1 shows the UV-vis absorption spectrum of a diluted<br />
solution of AgNPs <strong>in</strong> which we can see a narrow absorption at<br />
403 nm (plasmon band) <strong>in</strong>dicat<strong>in</strong>g the presence AgNPs. The<br />
size of the AgNPs were determ<strong>in</strong>ed with the help of<br />
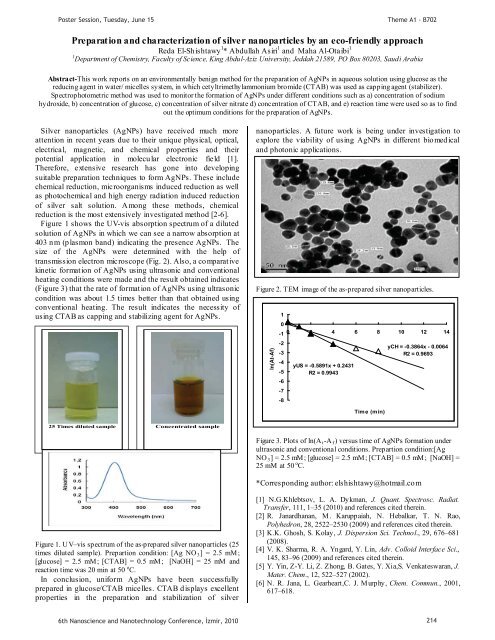
transmission electron microscope (Fig. 2). Also, a comparative<br />
k<strong>in</strong>etic formation of AgNPs us<strong>in</strong>g ultrasonic and conventional<br />
heat<strong>in</strong>g conditions were made and the result obta<strong>in</strong>ed <strong>in</strong>dicates<br />
(Figure 3) that the rate of formation of AgNPs us<strong>in</strong>g ultrasonic<br />
condition was about 1.5 times better than that obta<strong>in</strong>ed us<strong>in</strong>g<br />
conventional heat<strong>in</strong>g. The result <strong>in</strong>dicates the necessity of<br />
us<strong>in</strong>g CTAB as capp<strong>in</strong>g and stabiliz<strong>in</strong>g agent for AgNPs.<br />
nanoparticles. A future work is be<strong>in</strong>g under <strong>in</strong>vestigation to<br />
explore the viability of us<strong>in</strong>g AgNPs <strong>in</strong> different biomedical<br />
and photonic applications.<br />
Figure 2. TEM image of the as-prepared silver nanoparticles.<br />
ln(At-Af)<br />
1<br />
0<br />
-1 0 2 4 6 8 10 12 14<br />
-2<br />
-3<br />
-4<br />
-5<br />
-6<br />
-7<br />
-8<br />
yUS = -0.5891x + 0.2431<br />
R2 = 0.9943<br />
yCH = -0.3864x - 0.0064<br />
R2 = 0.9693<br />
Time (m<strong>in</strong>)<br />
25 Times diluted sample Concentrated sample<br />
Figure 3. Plots of ln(A t -A f ) versus time of AgNPs formation under<br />
ultrasonic and conventional conditions. Prepartion condition:[Ag<br />
NO 3 ] = 2.5 mM; [glucose] = 2.5 mM; [CTAB] = 0.5 mM; [NaOH] =<br />
25 mM at 50 o C.<br />
*Correspond<strong>in</strong>g author: elshishtawy@hotmail.com<br />
Figure 1. UV–vis spectrum of the as-prepared silver nanoparticles (25<br />
times diluted sample). Prepartion condition: [Ag NO 3 ] = 2.5 mM;<br />
[glucose] = 2.5 mM; [CTAB] = 0.5 mM; [NaOH] = 25 mM and<br />
reaction time was 20 m<strong>in</strong> at 50 o C.<br />
In conclusion, uniform AgNPs have been successfully<br />
prepared <strong>in</strong> glucose/CTAB micelles. CTAB displays excellent<br />
properties <strong>in</strong> the preparation and stabilization of silver<br />
[1] N.G.Khlebtsov, L. A. Dykman, J. Quant. Spectrosc. Radiat.<br />
Transfer, 111, 1–35 (2010) and references cited there<strong>in</strong>.<br />
[2] R. Janardhanan, M. Karuppaiah, N. Hebalkar, T. N. Rao,<br />
Polyhedron, 28, 2522–2530 (2009) and references cited there<strong>in</strong>.<br />
[3] K.K. Ghosh, S. Kolay, J. Dispersion Sci. Technol., 29, 676–681<br />
(2008).<br />
[4] V. K. Sharma, R. A. Yngard, Y. L<strong>in</strong>, Adv. Colloid Interface Sci.,<br />
145, 83–96 (2009) and references cited there<strong>in</strong>.<br />
[5] Y. Y<strong>in</strong>, Z-Y. Li, Z. Zhong, B. Gates, Y. Xia,S. Venkateswaran, J.<br />
Mater. Chem., 12, 522–527 (2002).<br />
[6] N. R. Jana, L. Gearheart,C. J. Murphy, Chem. Commun., 2001,<br />
617–618.<br />
6th Nanoscience and Nanotechnology Conference, zmir, 2010 214