Photonic crystals in biology
Photonic crystals in biology
Photonic crystals in biology
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Poster Session, Tuesday, June 15<br />
Theme A1 - B702<br />
Synthesis of SnO 2 Nanoparticles via Sol-Gel Method<br />
Hilal Kose 1 *, Emrah Bulut 1 1<br />
1 Department of Chemistry, Arts and Sciences Faculty, Sakarya University, 54187, Sakarya, Turkey<br />
Abstract- SnO 2 nanop articles fabricated at different pH values by sol-gel method. Sols were prepared from aqueous solution of t<strong>in</strong>(II)<br />
chloride at room temperature. SEM and XRD analysis were performed to characterize nanoparticles. The results <strong>in</strong>dicate the<br />
nanocrystall<strong>in</strong>e nature of the SnO 2 particles.<br />
T<strong>in</strong> oxide (SnO2), cassiterite structure, is a typical wide<br />
band gap n-type semiconductor (3.8 eV) [1] and, one of the<br />
most widely used semiconductor oxides due to its chemical<br />
and mechanical stabilit ies. The electrical and chemical<br />
properties of SnO 2 have been extensively studied because of<br />
its application as transparent electrodes for solar cells, liquid<br />
crystal displays; antistatic coat<strong>in</strong>gs and gas sensors; anodes<br />
for lithium ion batteries, transistors, catalyst supports; nano<br />
and ultra filtration membranes and anticorrosion coat<strong>in</strong>gs [2].<br />
Recently t<strong>in</strong> oxide-based materials have received<br />
considerable attention as promis<strong>in</strong>g anode materials for Liion<br />
batteries due to their high capacity [3]. SnO 2<br />
nanoparticles can be obta<strong>in</strong>ed by different techniques.<br />
Among the process<strong>in</strong>g techniques, sol-gel offers advantages<br />
such as low cost, low temperatures process<strong>in</strong>g and also<br />
precise control of stoichiometry [4].<br />
In this work, sol-gel method was applied to synthesize<br />
SnO 2 nanoparticles at different pH values. The SnO 2<br />
precursor solution was prepared from t<strong>in</strong> chloride by<br />
dissolv<strong>in</strong>g SnCl 2·2H 2 O <strong>in</strong> 50 ml of deionized water. The<br />
solution was <strong>in</strong> the form of turbid nature dur<strong>in</strong>g the first<br />
period of the dissolv<strong>in</strong>g and then the color was returned to a<br />
transparent nature when acetic acid was added. The addition<br />
of acetic acid was also aimed to prevent rapid hydrolysis for<br />
obta<strong>in</strong><strong>in</strong>g nucleation of nano sized species <strong>in</strong> the solution.<br />
After stirr<strong>in</strong>g a few hours, the solution pH was adjusted to 7,<br />
8, 9, and 10 by us<strong>in</strong>g ammonia solution (%25). Then the<br />
solution stirred for 24 h. The resultant sols washed and<br />
centrifuged 5 times with deionized water. F<strong>in</strong>ally, sols dried<br />
at 150 o C for 2 hours and precalc<strong>in</strong>ation was performed at<br />
300 o C. Subsequently, the calc<strong>in</strong>ation temperature was<br />
<strong>in</strong>creased to 400 o C and the sols were calc<strong>in</strong>ated for two<br />
hours at treatment.<br />
of spherical nanoparticles <strong>in</strong> diameter under 100 nm were<br />
obta<strong>in</strong>ed.<br />
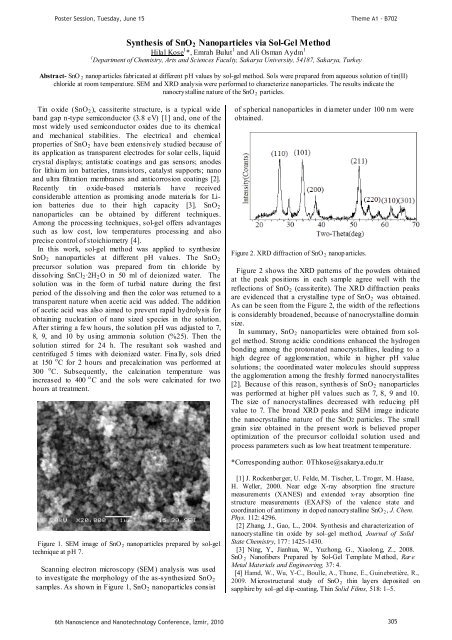
Figure 2. XRD diffraction of SnO 2 nanop articles.<br />
Figure 2 shows the XRD patterns of the powders obta<strong>in</strong>ed<br />
at the peak positions <strong>in</strong> each sample agree well with the<br />
reflections of SnO 2 (cassiterite). The XRD diffraction peaks<br />
are evidenced that a crystall<strong>in</strong>e type of SnO 2 was obta<strong>in</strong>ed.<br />
As can be seen from the Figure 2, the width of the reflections<br />
is considerably broadened, because of nanocrystall<strong>in</strong>e do ma<strong>in</strong><br />
size.<br />
In summary, SnO 2 nanoparticles were obta<strong>in</strong>ed from solgel<br />
method. Strong acidic conditions enhanced the hydrogen<br />
bond<strong>in</strong>g among the protonated nanocrystallites, lead<strong>in</strong>g to a<br />
high degree of agglomeration, while <strong>in</strong> higher pH value<br />
solutions; the coord<strong>in</strong>ated water molecules should suppress<br />
the agglomeration among the freshly formed nanocrystallites<br />
[2]. Because of this reason, synthesis of SnO 2 nanoparticles<br />
was performed at higher pH values such as 7, 8, 9 and 10.<br />
The size o f nanocrystall<strong>in</strong>es decreased with reduc<strong>in</strong>g pH<br />
value to 7. The broad XRD peaks and SEM image <strong>in</strong>dicate<br />
the nanocrystall<strong>in</strong>e nature of the SnO2 particles. The small<br />
gra<strong>in</strong> size obta<strong>in</strong>ed <strong>in</strong> the present work is believed proper<br />
optimization of the precursor colloidal solution used and<br />
process parameters such as low heat treatment temperature.<br />
*Correspond<strong>in</strong>g author: 0Thkose@sakarya.edu.tr<br />
Figure 1. SEM image of SnO 2 nanop articles prepared by sol-gel<br />
technique at pH 7.<br />
Scann<strong>in</strong>g electron microscopy (SEM) analysis was used<br />
to <strong>in</strong>vestigate the morphology of the as-synthesized SnO2<br />
samples. As shown <strong>in</strong> Figure 1, SnO 2 nanoparticles consist<br />
[1] J. Rockenberger, U. Felde, M. Tischer, L. Troger, M. Haase,<br />
H. Weller, 2000. Near edge X-ray absorption f<strong>in</strong>e structure<br />
measurements (XANES) and extended x-ray absorption f<strong>in</strong>e<br />
structure measurements (EXAFS) of the valence state and<br />
coord<strong>in</strong>ation of antimony <strong>in</strong> doped nanocrystall<strong>in</strong>e SnO 2 , J. Chem.<br />
Phys. 112: 4296.<br />
[2] Zhang, J., Gao, L., 2004. Synthesis and characterization of<br />
nanocrystall<strong>in</strong>e t<strong>in</strong> oxide by sol–gel method, Journal of Solid<br />
State Chemistry, 177: 1425-1430.<br />
[3] N<strong>in</strong>g, Y., Jianhua, W., Yuzhong, G., Xiaolong, Z., 2008.<br />
SnO 2 Nanofibers Prepared by Sol-Gel Template Method, Rar e<br />
Metal Materials and Eng<strong>in</strong>eer<strong>in</strong>g, 37: 4.<br />
[4] Hamd, W., Wu, Y-C., Boulle, A., Thune, E., Gu<strong>in</strong>ebretière, R.,<br />
2009. Microstructural study of SnO 2 th<strong>in</strong> layers deposited on<br />
sapphire by sol–gel dip-coat<strong>in</strong>g, Th<strong>in</strong> Solid Films, 518: 1–5.<br />
6th Nanoscience and Nanotechnology Conference, zmir, 2010 305