Photonic crystals in biology
Photonic crystals in biology
Photonic crystals in biology
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Poster Session, Tuesday, June 15<br />
Theme A1 - B702<br />
Investigation of nucleation and growth mechanism dur<strong>in</strong>g formation of poly(azure A)<br />
Emrah Kalyoncu, Kader Dacı, brahim Hakkı Kaplan, Ezgi Topçu, Murat Alanyalıolu*<br />
Department of Chemistry, Sciences Faculty, Atatürk University, Erzurum 25240, Turkey<br />
Abstract—Nucleation and growth mechanism dur<strong>in</strong>g formation of poly(AA) films on gold substrates was <strong>in</strong>testigated. Repeated potential<br />
cycl<strong>in</strong>g by us<strong>in</strong>g cyclic voltammetry and potential controlled electrolysis techniques have been performed to synthesize poly(AA) th<strong>in</strong> films on<br />
gold work<strong>in</strong>g electrodes <strong>in</strong> the solution conta<strong>in</strong><strong>in</strong>g 0.1 mM AA and 0.1 M phosphate solution (pH:6.2). Chronoamperometry, STM (Scann<strong>in</strong>g<br />
Tunnel<strong>in</strong>g Microscopy), AFM (Atomic Force Microscopy), and UV-vis. absorption spectroscopy techniques were applied for the characterization<br />
of prepared polymeric films.<br />
Dye polymer films show excellent catalytic and<br />
photoelectrochemical properties and have been applied for<br />
batteries and electrodes, electrochromic devices, light emitt<strong>in</strong>g<br />
diodes, and immobilizitaion of enzymes [1,2]. Dye polymer<br />
films must have a well-ordered surface to be used <strong>in</strong> these<br />
technological applications. Electropolymerization is one of the<br />
simple and useful method to obta<strong>in</strong> dye polymer films. In the<br />
electropolymerization process, deposition of polymeric dye<br />
film on the electrode surface is achieved by constant or cycled<br />
potential oxidation of a dye-conta<strong>in</strong><strong>in</strong>g solution. Azure A is a<br />
derivative of phenothiaz<strong>in</strong>e dye material (Figure 1).<br />
for progressive case. Growth of nuclei is slow for<br />
<strong>in</strong>stantaneous nucleation and fast for progressive nucleation<br />
[7,8]. Accord<strong>in</strong>g to Li and Albery [9], two possible<br />
mechanisms are possible for polymer films: Progressive twodimensional<br />
layer-by-layer nucleation and <strong>in</strong>stantaneous threedimensional<br />
nucleation and growth. In this study,<br />
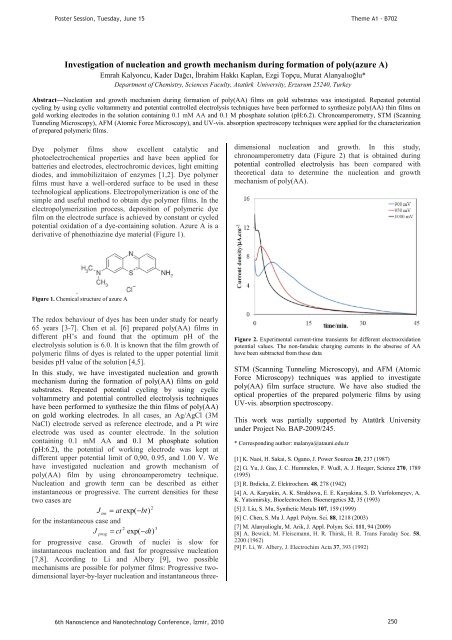
chronoamperometry data (Figure 2) that is obta<strong>in</strong>ed dur<strong>in</strong>g<br />
potential controlled electrolysis has been compared with<br />
theoretical data to determ<strong>in</strong>e the nucleation and growth<br />
mechanism of poly(AA).<br />
.<br />
Figure 1. Chemical structure of azure A<br />
The redox behaviour of dyes has been under study for nearly<br />
65 years [3-7]. Chen et al. [6] prepared poly(AA) films <strong>in</strong><br />
different pH’s and found that the optimum pH of the<br />
electrolysis solution is 6.0. It is known that the film growth of<br />
polymeric films of dyes is related to the upper potential limit<br />
besides pH value of the solution [4,5].<br />
In this study, we have <strong>in</strong>vestigated nucleation and growth<br />
mechanism dur<strong>in</strong>g the formation of poly(AA) films on gold<br />
substrates. Repeated potential cycl<strong>in</strong>g by us<strong>in</strong>g cyclic<br />
voltammetry and potential controlled electrolysis techniques<br />
have been performed to synthesize the th<strong>in</strong> films of poly(AA)<br />
on gold work<strong>in</strong>g electrodes. In all cases, an Ag/AgCl (3M<br />
NaCl) electrode served as reference electrode, and a Pt wire<br />
electrode was used as counter electrode. In the solution<br />
conta<strong>in</strong><strong>in</strong>g 0.1 mM AA and 0.1 M phosphate solution<br />
(pH:6.2), the potential of work<strong>in</strong>g electrode was kept at<br />
different upper potential limit of 0,90, 0.95, and 1.00 V. We<br />
have <strong>in</strong>vestigated nucleation and growth mechanism of<br />
poly(AA) film by us<strong>in</strong>g chronoamperometry technique.<br />
Nucleation and growth term can be described as either<br />
<strong>in</strong>stantaneous or progressive. The current densities for these<br />
two cases are<br />
2<br />
J <strong>in</strong>s<br />
= at exp( −bt)<br />
for the <strong>in</strong>stantaneous case and<br />
2<br />
3<br />
= ct exp( −dt)<br />
J prog<br />
Figure 2. Experimental current-time transients for different electrooxidation<br />
potential values. The non-faradaic charg<strong>in</strong>g currents <strong>in</strong> the absense of AA<br />
have been subtracted from these data<br />
STM (Scann<strong>in</strong>g Tunnel<strong>in</strong>g Microscopy), and AFM (Atomic<br />
Force Microscopy) techniques was applied to <strong>in</strong>vestigate<br />
poly(AA) film surface structure. We have also studied the<br />
optical properties of the prepared polymeric films by us<strong>in</strong>g<br />
UV-vis. absorption spectroscopy.<br />
This work was partially supported by Atatürk University<br />
under Project No. BAP-2009/245.<br />
* Correspond<strong>in</strong>g author: malanya@atauni.edu.tr<br />
[1] K. Naoi, H. Sakai, S. Ogano, J. Power Sources 20, 237 (1987)<br />
[2] G. Yu, J. Gao, J. C. Hummelen, F. Wudl, A. J. Heeger, Science 270, 1789<br />
(1995)<br />
[3] R. Brdicka, Z. Elektrochem. 48, 278 (1942)<br />
[4] A. A. Karyak<strong>in</strong>, A. K. Strakhova, E. E. Karyak<strong>in</strong>a, S. D. Varfolomeyev, A.<br />
K. Yatsimirsky, Bioelectrochem. Bioenergetics 32, 35 (1993)<br />
[5] J. Liu, S. Mu, Synthetic Metals 107, 159 (1999)<br />
[6] C. Chen, S. Mu J. Appl. Polym. Sci. 88, 1218 (2003)<br />
[7] M. Alanyalioglu, M. Arik, J. Appl. Polym. Sci. 111, 94 (2009)<br />
[8] A. Bewick, M. Fleiscmann, H. R. Thirsk, H. R. Trans Faraday Soc. 58,<br />
2200 (1962)<br />
[9] F. Li, W. Albery, J. Electrochim Acta 37, 393 (1992)<br />
6th Nanoscience and Nanotechnology Conference, zmir, 2010 250