PP andPoster Session, Thursday, June 17Theme F686 - N11231Poly(V<strong>in</strong>yl Chloride)/Kaol<strong>in</strong>ite Nanocomposites111UYasem<strong>in</strong> TurhanUP P*, Mehmet DoanP Mahir AlkanPPBalikesir University, Faculty of Science and Literature, Department of Chemistry, 10145 Balikesir, TurkeyAbstract- Nanocomposites of poly(v<strong>in</strong>yl chloride) (PVC) have been prepared by solution <strong>in</strong>tercalation method us<strong>in</strong>g both natural andmodified kaol<strong>in</strong>ites. Kaol<strong>in</strong>ite was modified with dimethyl sulfoxide (DMSO) to expand the <strong>in</strong>terlayer basal spac<strong>in</strong>g. The characterization ofPVC/kaol<strong>in</strong>ite nanocomposites was made by X-ray diffraction (XRD) and transmission electron microscopy (TEM); the <strong>in</strong>teractions betweenkaol<strong>in</strong>ite and PVC was discussed by FTIR-ATR; the thermal stability was determ<strong>in</strong>ed by simultaneous DTA/TG. FTIR-ATR confirmshydrogen bonds formed between dimethyl sulfoxide molecules and the <strong>in</strong>ner surface hydroxyl groups of kaol<strong>in</strong>ite. XRD and TEM resultsgive evidence that kaol<strong>in</strong>ite was dramatically <strong>in</strong>tercalated <strong>in</strong>to nanoscale and homogenously dispersed <strong>in</strong> the PVC matrix. Thermogravimetricanalysis <strong>in</strong>dicated that <strong>in</strong>troduction of clay to the polymer network resulted <strong>in</strong> an <strong>in</strong>crease <strong>in</strong> thermal stability. Ultraviolet (UV) absorbanceexperiments showed that nanocomposites have a higher UV transmission than PVC film.The synthesis and characterization of new and novelmaterials are one of the ma<strong>in</strong> objectives of advancedmaterial research. Polymer nanocomposites, especiallypolymer-layered silicate nanocomposites, have become avaluable alternative to conventionally filled polymers andare of current <strong>in</strong>terest because of the fundamental questionsthey address and their potential technologicalapplications.[1-4]In this study, we synthesed nanocomposites with differentrelative compositions based on PVC and both natural andmodified kaol<strong>in</strong>ites by solution <strong>in</strong>tercalation method.Modified kaol<strong>in</strong>it was prepared with succunimide via questdisplacementreaction. Figures 1, 2 and 3 show thesereactions.the formation of residue and improve the thermal stability ofthe polymer matrix. The <strong>in</strong>tercalated composites exhibitbigger UV transparency, but this transparency decreases with<strong>in</strong>crease <strong>in</strong> kaol<strong>in</strong>ite amount. TEM results have showed thatthe nanocomposites have both <strong>in</strong>tercalated and exfloitedmorphology as shown Figure 4.Figure 1. Quest-displacement reactionFigure 4. Process<strong>in</strong>g of nanocomposite and tem image of clay andnanocomposite0,71nm +Ultra saund field120 h stirr<strong>in</strong>g1,11 nmThe work was f<strong>in</strong>ancially supported by Balikesir UniversityResearch Fund (Project 2008/20).DMSOFigure 2. Intercalation of kaol<strong>in</strong>ite with DMSO.( :O, :H, :S,: C )Figure 3. Quest-displacement reaction of DMSO betweenSIM.( :O, :H, :S , :N, :C )As a result, a series of nanocomposite materials consist<strong>in</strong>gof PVC and layered kaol<strong>in</strong>ite clay were prepared byeffectively dispers<strong>in</strong>g of the <strong>in</strong>organic nanolayers ofkaol<strong>in</strong>ite clay <strong>in</strong> PVC matrix by the solution <strong>in</strong>tercalationmethod FTIR-ATR, XRD, TEM, DTA/TG, BET and UV-Vis spectrophotometer experiments were carried out tocharacterize the morphology and properties of thenanocomposites. By means of <strong>in</strong>tercalation of kaol<strong>in</strong>ite withDMSO, the basal spac<strong>in</strong>g of a natural kaol<strong>in</strong>ite expandedfrom 0.71 to 1.11 nm as shown <strong>in</strong> Figure 2. It has also beenobserved that the organophilicity of kaol<strong>in</strong>ite was enhanced.The <strong>in</strong>tercalation of KDMSO with SIM are <strong>in</strong>tercalated <strong>in</strong>the <strong>in</strong>terlayer spaces of kaol<strong>in</strong>ite by guest-displacementmethod as shown <strong>in</strong> Figure 3.Evidence from several spectroscopic and thermalanalysis shows that SIM replaces the DMSO molecules. The<strong>in</strong>corporation of nanoparticle with polymer results <strong>in</strong> an<strong>in</strong>crease <strong>in</strong> thermal stability. The nanocomposites enhance*Correspond<strong>in</strong>g author:yozdemir@balikesir.edu.tr[1] P<strong>in</strong>navaia, T.J., Beall, G.W.,2000. Polymer-ClayNanocomposites. United K<strong>in</strong>gdom, U.K: Wiley Series <strong>in</strong> PolymerScience; Wiley Chichester.[2] Viville, P., Lazzaroni, R., Pollet, E., Alexandre, M., Dubois, P.,Borcia, G., Pireaux, J. J.,2003. Surface characterization of poly(_-caprolactone)-based nanocomposites,, Langmuir, 19: 9425–9433.[3] Alexandre, M., Dubois, P., 2000. Polymer-layered silicatenanocomposites:Preparation, properties, and uses of a new class ofmaterials,, Mater. Sci.Eng., 28 (1-2): 1–63.[4] Turhan, Y., Doan, M.,Alkan, M., 2010. Poly(v<strong>in</strong>ylchloride)/Kaol<strong>in</strong>ite Nanocomposites: Characterization and Thermaland Optical Properties,, Ind. Eng. Chem. Res.,49: 1503-1513.6th Nanoscience and Nanotechnology Conference, zmir, 2010 725
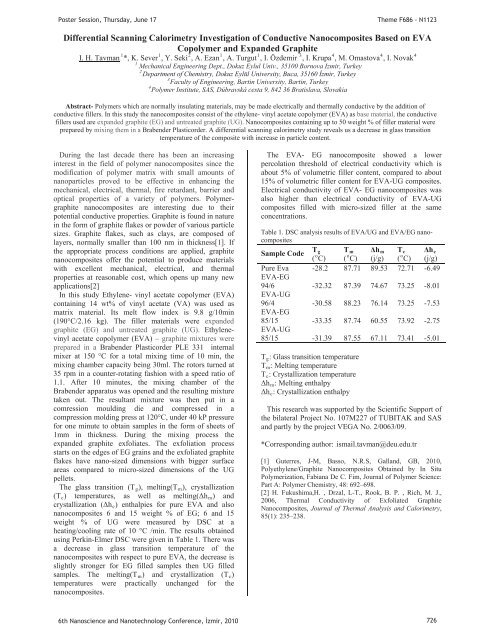
PPPPP,PP,P(PR RmPoster Session, Thursday, June 17Theme F686 - N1123Differential Scann<strong>in</strong>g Calorimetry Investigation of Conductive Nanocomposites Based on EVACopolymer and Expanded Graphite112113444UI. H. TavmanUP P*, K. SeverP P, Y. SekiP P, A. EzanPPA. TurgutPPI. Özdemir P P, I. KrupaP P, M. OmastovaP P, I. NovakP1PMechanical Eng<strong>in</strong>eer<strong>in</strong>g Dept., Dokuz Eylul Univ., 35100 Bornova Izmir, Turkey2PTDepartment of Chemistry, Dokuz Eylül University, Buca, 35160 zmir, TurkeyPFaculty of Eng<strong>in</strong>eer<strong>in</strong>g, Bart<strong>in</strong> University, Bart<strong>in</strong>, TurkeyPPolymer Institute, SAS, Dúbravská cesta 9, 842 36 Bratislava, Slovakia43Abstract- Polymers which are normally <strong>in</strong>sulat<strong>in</strong>g materials, may be made electrically and thermally conductive by the addition ofconductive fillers. In this study the nanocomposites consist of the ethylene- v<strong>in</strong>yl acetate copolymer (EVA) as base material, the conductivefillers used are expanded graphite (EG) and untreated graphite (UG). Nanocomposites conta<strong>in</strong><strong>in</strong>g up to 50 weight % of filler material wereprepared by mix<strong>in</strong>g them <strong>in</strong> a Brabender Plasticorder. A differential scann<strong>in</strong>g calorimetry study reveals us a decrease <strong>in</strong> glass transitiontemperature of the composite with <strong>in</strong>crease <strong>in</strong> particle content.Dur<strong>in</strong>g the last decade there has been an <strong>in</strong>creas<strong>in</strong>g<strong>in</strong>terest <strong>in</strong> the field of polymer nanocomposites s<strong>in</strong>ce themodification of polymer matrix with small amounts ofnanoparticles proved to be effective <strong>in</strong> enhanc<strong>in</strong>g themechanical, electrical, thermal, fire retardant, barrier andoptical properties of a variety of polymers. Polymergraphitenanocomposites are <strong>in</strong>terest<strong>in</strong>g due to theirpotential conductive properties. Graphite is found <strong>in</strong> nature<strong>in</strong> the form of graphite flakes or powder of various particlesizes. Graphite flakes, such as clays, are composed oflayers, normally smaller than 100 nm <strong>in</strong> thickness[1]. Ifthe appropriate process conditions are applied, graphitenanocomposites offer the potential to produce materialswith excellent mechanical, electrical, and thermalproperties at reasonable cost, which opens up many newapplications[2]In this study Ethylene- v<strong>in</strong>yl acetate copolymer (EVA)conta<strong>in</strong><strong>in</strong>g 14 wt% of v<strong>in</strong>yl acetate (VA) was used asmatrix material. Its melt flow <strong>in</strong>dex is 9.8 g/10m<strong>in</strong>(190°C/2.16 kg). The filler materials were expandedgraphite (EG) and untreated graphite (UG). Ethylenev<strong>in</strong>ylacetate copolymer (EVA) – graphite mixtures wereprepared <strong>in</strong> a Brabender Plasticorder PLE 331 <strong>in</strong>ternalmixer at 150 °C for a total mix<strong>in</strong>g time of 10 m<strong>in</strong>, themix<strong>in</strong>g chamber capacity be<strong>in</strong>g 30ml. The rotors turned at35 rpm <strong>in</strong> a counter-rotat<strong>in</strong>g fashion with a speed ratio of1.1. After 10 m<strong>in</strong>utes, the mix<strong>in</strong>g chamber of theBrabender apparatus was opened and the result<strong>in</strong>g mixturetaken out. The resultant mixture was then put <strong>in</strong> acomression mould<strong>in</strong>g die and compressed <strong>in</strong> acompression mold<strong>in</strong>g press at 120°C, under 40 kP pressurefor one m<strong>in</strong>ute to obta<strong>in</strong> samples <strong>in</strong> the form of sheets of1mm <strong>in</strong> thickness. Dur<strong>in</strong>g the mix<strong>in</strong>g process theexpanded graphite exfoliates. The exfoliation processstarts on the edges of EG gra<strong>in</strong>s and the exfoliated graphiteflakes have nano-sized dimensions with bigger surfaceareas compared to micro-sized dimensions of the UGpellets.The glass transition (TRgR), melt<strong>in</strong>g(TRmR), crystallization(TRcR) temperatures, as well as melt<strong>in</strong>g(hRmR) andcrystallization (hRcR) enthalpies for pure EVA and alsonanocomposites 6 and 15 weight % of EG; 6 and 15weight % of UG were measured by DSC at aheat<strong>in</strong>g/cool<strong>in</strong>g rate of 10 °C /m<strong>in</strong>. The results obta<strong>in</strong>edus<strong>in</strong>g Perk<strong>in</strong>-Elmer DSC were given <strong>in</strong> Table 1. There wasa decrease <strong>in</strong> glass transition temperature of thenanocomposites with respect to pure EVA, the decrease isslightly stronger for EG filled samples then UG filledsamples. The melt<strong>in</strong>g(TRmR) and crystallization (TRcR)temperatures were practically unchanged for thenanocomposites.The EVA- EG nanocomposite showed a lowerpercolation threshold of electrical conductivity which isabout 5% of volumetric filler content, compared to about15% of volumetric filler content for EVA-UG composites.Electrical conductivity of EVA- EG nanocomposites wasalso higher than electrical conductivity of EVA-UGcomposites filled with micro-sized filler at the sameconcentrations.Table 1. DSC analysis results of EVA/UG and EVA/EG nanocompositesT g T hRm TRc hRcSample Code oooPC) (P PC) (j/g) (P PC) (j/g)Pure Eva -28.2 87.71 89.53 72.71 -6.49EVA-EG94/6 -32.32 87.39 74.67 73.25 -8.01EVA-UG96/4 -30.58 88.23 76.14 73.25 -7.53EVA-EG85/15 -33.35 87.74 60.55 73.92 -2.75EVA-UG85/15 -31.39 87.55 67.11 73.41 -5.01TRgR: Glass transition temperatureTRmR: Melt<strong>in</strong>g temperatureTRcR: Crystallization temperaturehRmR: Melt<strong>in</strong>g enthalpyhRcR: Crystallization enthalpyThis research was supported by the Scientific Support ofthe bilateral Project No. 107M227 of TUBITAK and SASand partly by the project VEGA No. 2/0063/09.*Correspond<strong>in</strong>g author: HTismail.tavman@deu.edu.trT[1] Guterres, J-M, Basso, N.R.S, Galland, GB, 2010,Polyethylene/Graphite Nanocomposites Obta<strong>in</strong>ed by In SituPolymerization, Fabiana De C. Fim, Journal of Polymer Science:Part A: Polymer Chemistry, 48: 692–698.[2] H. Fukushima,H. , Drzal, L-T., Rook, B. P. , Rich, M. J.,2006, Thermal Conductivity of Exfoliated GraphiteNanocomposites, Journal of Thermal Analysis and Calorimetry,85(1): 235–238.6th Nanoscience and Nanotechnology Conference, zmir, 2010 726
- Page 1:
Poster Presentations3rd Day17 June
- Page 4 and 5:
Determination of Dielectric Anisotr
- Page 7 and 8:
Poster Session, Thursday, June 17Th
- Page 9 and 10:
PP mPP vs.P =P,PP (1)P andPoster Se
- Page 11 and 12:
PP mPP vs.P =P,PP (1)P andPoster Se
- Page 13 and 14:
PP andPoster Session, Thursday, Jun
- Page 15 and 16:
Poster Session, Thursday, June 17Th
- Page 17 and 18:
PP and770 772 774 776 778 780 782 7
- Page 19 and 20:
Poster Session, Thursday, June 17Th
- Page 21 and 22:
Poster Session, Thursday, June 17Th
- Page 23 and 24:
P25,Poster Session, Thursday, June
- Page 25 and 26:
PP TOBBPoster Session, Thursday, Ju
- Page 27 and 28:
PisPPisisisP,PisPoster Session, Thu
- Page 29 and 30:
U NeslihanPPPPoster Session, Thursd
- Page 31 and 32:
Poster Session, Thursday, June 17Th
- Page 33 and 34:
PPPoster Session, Thursday, June 17
- Page 35 and 36:
PPoster Session, Thursday, June 17T
- Page 37 and 38:
P onP viaPP wereP upPoster Session,
- Page 39 and 40:
P ·cm.PVPPPsPPPPP andPoster Sessio
- Page 41 and 42:
Poster Session, Thursday, June 17Th
- Page 43 and 44:
PPoster Session, Thursday, June 17T
- Page 45 and 46:
PPoster Session, Thursday, June 17T
- Page 47 and 48:
Poster Session, Thursday, June 17Th
- Page 49 and 50:
PErkanPoster Session, Thursday, Jun
- Page 51 and 52:
Poster Session, Thursday, June 17Th
- Page 53 and 54:
Poster Session, Thursday, June 17Th
- Page 55 and 56:
PPPP andPoster Session, Thursday, J
- Page 57 and 58:
Poster Session, Thursday, June 17Th
- Page 59 and 60:
Poster Session, Thursday, June 17Th
- Page 61 and 62:
T PeptideTPP,PP,PP andTT2429TTTTTT
- Page 63 and 64:
Poster Session, Thursday, June 17Th
- Page 65 and 66: PPoster Session, Thursday, June 17T
- Page 67 and 68: Poster Session, Thursday, June 17Th
- Page 69 and 70: PPPoster Session, Thursday, June 17
- Page 71 and 72: Poster Session, Thursday, June 17Th
- Page 73 and 74: Poster Session, Thursday, June 17Th
- Page 75 and 76: PT AdditionalT ThePoster Session, T
- Page 77 and 78: Poster Session, Thursday, June 17Th
- Page 79 and 80: Poster Session, Thursday, June 17Th
- Page 81 and 82: Poster Session, Thursday, June 17Th
- Page 83 and 84: PPoster Session, Thursday, June 17T
- Page 85 and 86: Poster Session, Thursday, June 17Th
- Page 87 and 88: PPPoster Session, Thursday, June 17
- Page 89 and 90: Poster Session, Thursday, June 17Hu
- Page 91 and 92: Poster Session, Thursday, June 17Th
- Page 93 and 94: PPPPPPoster Session, Thursday, June
- Page 95 and 96: Poster Session, Thursday, June 17Th
- Page 97 and 98: Poster Session, Thursday, June 17Th
- Page 99 and 100: Poster Session, Thursday, June 17Th
- Page 101 and 102: PPoster Session, Thursday, June 17T
- Page 103 and 104: Poster Session, Thursday, June 17Th
- Page 105 and 106: PPPPPPPoster Session, Thursday, Jun
- Page 107 and 108: Poster Session, Thursday, June 17Th
- Page 109 and 110: PPPR2R PIN(80)PPgPP OzlemPPoster Se
- Page 111 and 112: Poster Session, Thursday, June 17Th
- Page 113 and 114: Poster Session, Thursday, June 17Th
- Page 115: P onPP toP coordinatedPPoster Sessi
- Page 119 and 120: Poster Session, Thursday, June 17Th
- Page 121 and 122: Poster Session, Thursday, June 17Th
- Page 123 and 124: PP InstitutePP DepartmentPoster Ses
- Page 125 and 126: andPCPPoster Session, Thursday, Jun
- Page 127 and 128: PP scatteringPYusufPP Corresponding
- Page 129 and 130: PP toPoster Session, Thursday, June
- Page 131 and 132: PP andPoster Session, Thursday, Jun
- Page 133 and 134: PPPPoster Session, Thursday, June 1
- Page 135 and 136: PPoster Session, Thursday, June 17T
- Page 137 and 138: PPP andP (.cm).Poster Session, Thur
- Page 139 and 140: PP tiltP andP editionPoster Session
- Page 141 and 142: PP andPPoster Session, Thursday, Ju
- Page 143 and 144: Poster Session, Thursday, June 17Th
- Page 145 and 146: PP forP forP edit.PPoster Session,
- Page 147 and 148: Poster Session, Thursday, June 17Th
- Page 149 and 150: Poster Session, Thursday, June 17Th
- Page 151 and 152: PP ionicPP ,PPoster Session, Thursd
- Page 153 and 154: PP lightPoster Session, Thursday, J
- Page 155 and 156: Poster Session, Thursday, June 17Th
- Page 157 and 158: PPoster Session, Thursday, June 17T
- Page 159 and 160: Poster Session, Thursday, June 17Th
- Page 161 and 162: PandPoster Session, Thursday, June
- Page 163 and 164: Poster Session, Thursday, June 17 T
- Page 165 and 166: PPPoster Session, Thursday, June 17
- Page 167 and 168:
PPoster Session, Thursday, June 17T
- Page 169 and 170:
PPoster Session, Thursday, June 17T
- Page 171 and 172:
PPoster Session, Thursday, June 17T
- Page 173 and 174:
PP DepartmentNanoscienceTPPoster Se
- Page 175 and 176:
Poster Session, Thursday, June 17Th
- Page 177 and 178:
Poster Session, Thursday, June 17Th
- Page 179 and 180:
PPPoster Session, Thursday, June 17
- Page 181 and 182:
PPPPPoster Session, Thursday, June
- Page 183 and 184:
PPPPoster Session, Thursday, June 1
- Page 185 and 186:
PPoster Session, Thursday, June 17T
- Page 187 and 188:
PPoster Session, Thursday, June 17T
- Page 189 and 190:
PPoster Session, Thursday, June 17T
- Page 191 and 192:
Poster Session, Thursday, June 17Th
- Page 193 and 194:
Poster Session, Thursday, June 17Th
- Page 195 and 196:
0T0T0T0T AsPPPP werePoster Session,
- Page 197 and 198:
PPoster Session, Thursday, June 17T
- Page 199 and 200:
PPPPPoster Session, Thursday, June
- Page 201 and 202:
PPoster Session, Thursday, June 17T
- Page 203 and 204:
PPoster Session, Thursday, June 17T
- Page 205 and 206:
Poster Session, Thursday, June 17Th
- Page 207 and 208:
PPoster Session, Thursday, June 17T
- Page 209 and 210:
PPoster Session, Thursday, June 17T
- Page 211:
Poster Session, Thursday, June 17AF