PPmaximumPoster Session, Thursday, June 17Theme F686 - N1123An experiment <strong>in</strong>vestigation of GaAs/AlGaAs Solar Cells Efficiency11122ULeyla Baak BüklüUP P*, Aye ErolP P, M. Çet<strong>in</strong> ArikanP P, Ben RoyallPPand Naci BalkanP1PDepartment of Physics, Istanbul University, Istanbul, TurkeyPDepartment of Comput<strong>in</strong>g and Electronic Systems, Essex University, UK2Abstract- In this work, the efficiency of GaAs/AlGaAs solar cell which was formed by a top r<strong>in</strong>g geometry by photolithography methodobta<strong>in</strong>ed. The efficiency and spectral response of the cells were <strong>in</strong>vestigated by I-V measurement and photoconductivity (PC) measurementsrespectively.Solar cell devices based on III-V semiconductors have anefficiency around 30% which is higher than Si based cells.Because the broader part of solar spectrum is covered bythese structures [1]. The band gap of ternary and quaternaryIII-V semiconductors can be tailored chang<strong>in</strong>g alloycompositions for example the band gap energy of GaR1-xRAlRxRAssemiconductors changes from 1,42eV (GaAs) to 2,16eV(AlAs).In this study epitaxially grown n on p GaAs/GaAlAs solarcell structure given <strong>in</strong> Figure 1 has been employed. GaAlAshav<strong>in</strong>g Al concentration of 0,8 was added to the structure <strong>in</strong>order to absorb solar energy start<strong>in</strong>g from 1,6 eV.0,100,080,06Figure 3. I-V set-up3cm8cm13cm18cm23cm28cm33cmI (mA)0,040,020,00-0,020,0 0,1 0,2 0,3 0,4 0,5 0,6 0,7V(Volt)Figure 1. The solar cell structureSolar cell structure was fabricated <strong>in</strong> r<strong>in</strong>g contacts as mesastructures hav<strong>in</strong>g contacts on the top of the structures us<strong>in</strong>gphotolithography techniques as shown <strong>in</strong> Figure 2.Top layer was just for ohmic contacts and <strong>in</strong>terior part ofthe r<strong>in</strong>g removed after r<strong>in</strong>g shaped gold deposition byselectively etch<strong>in</strong>g. Au/Ge:Au/Ni alloy was used as ohmiccontact to n type material on the top, Au:Zn/Au ohmiccontact to p type material at the bottom.Figure 4. I-V characteristic of solar cell has taken depend<strong>in</strong>g on thedistance between sample and UV lampThe efficiency of solar cell calculated us<strong>in</strong>g the equationgiven below.IscVocFF (1)PsWhere R PRs, power; FF, fill factor; IRscR, shortcircuitcurrent; VRoc, Ropen-circuit voltage. Us<strong>in</strong>g Equation 1,the efficiency of the solar cell calculated as 13%.5 35K43PC(a.u.)2Figure 2. Solar cell which formed by photolithographyFabricated structure was mounted on a ceramic holder withgold contact pads that was used for wir<strong>in</strong>g. Sample contactsfrom the top and from the bottom layers were jo<strong>in</strong> to goldpaths by ultrasonic bond<strong>in</strong>g.I-V measurements were taken <strong>in</strong> a light tight box with200W high pressure mercury lamp as shown <strong>in</strong> Figure 3.Obta<strong>in</strong>ed spectrum was taken at different <strong>in</strong>tensities bychang<strong>in</strong>g distance between light source and solar cell. Figure4 shows the obta<strong>in</strong>ed I-V curves were taken at different light<strong>in</strong>tensities by us<strong>in</strong>g <strong>in</strong>verse square law.101,2 1,4 1,6 1,8 2,0 2,2 2,4 2,6 2,8 3,0 3,2Energy(eV)Figure 5. Photoconductivity measurement of solar cell has taken35K, 100mV sensitivity and 100Hz frequencyAs seen from the Figure 5, PC spectrum of solar cell startsabsorb<strong>in</strong>g at 1,5eV and peaks at 1.65eV.In summary, the efficiency of GaAs/AlGaAs structurecalculated from I-V curve and spectral response region wasobta<strong>in</strong>ed.*Correspond<strong>in</strong>g author: lbbuklu@gmail.com[1] A.W. Bett1, F. Dimroth2, G. Stollwerck2, O.V. Sulima,1999, Appl. Phys. A 69, 119–1296th Nanoscience and Nanotechnology Conference, zmir, 2010 652
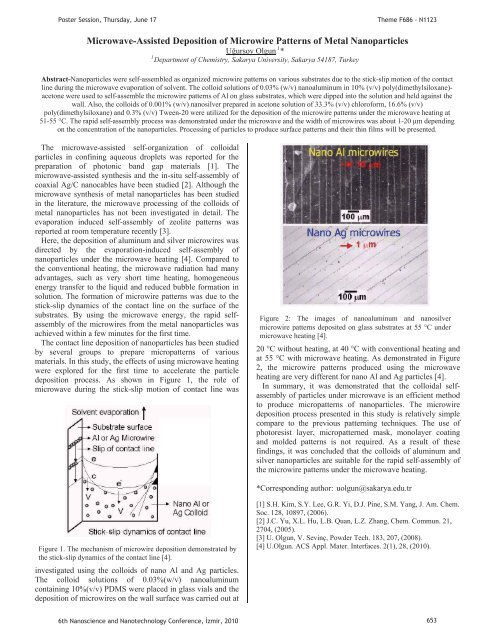
PPoster Session, Thursday, June 17Theme F686 - N1123Microwave-Assisted Deposition of Microwire Patterns of Metal Nanoparticles1UUursoy OlgunUP P*1PDepartment of Chemistry, Sakarya University, Sakarya 54187, TurkeyAbstract-Nanoparticles were self-assembled as organized microwire patterns on various substrates due to the stick-slip motion of the contactl<strong>in</strong>e dur<strong>in</strong>g the microwave evaporation of solvent. The colloid solutions of 0.03% (w/v) nanoalum<strong>in</strong>um <strong>in</strong> 10% (v/v) poly(dimethylsiloxane)-acetone were used to self-assemble the microwire patterns of Al on glass substrates, which were dipped <strong>in</strong>to the solution and held aga<strong>in</strong>st thewall. Also, the colloids of 0.001% (w/v) nanosilver prepared <strong>in</strong> acetone solution of 33.3% (v/v) chloroform, 16.6% (v/v)poly(dimethylsiloxane) and 0.3% (v/v) Tween-20 were utilized for the deposition of the microwire patterns under the microwave heat<strong>in</strong>g at51-55 °C. The rapid self-assembly process was demonstrated under the microwave and the width of microwires was about 1-20 m depend<strong>in</strong>gon the concentration of the nanoparticles. Process<strong>in</strong>g of particles to produce surface patterns and their th<strong>in</strong> films will be presented.The microwave-assisted self-organization of colloidalparticles <strong>in</strong> conf<strong>in</strong><strong>in</strong>g aqueous droplets was reported for thepreparation of photonic band gap materials [1]. Themicrowave-assisted synthesis and the <strong>in</strong>-situ self-assembly ofcoaxial Ag/C nanocables have been studied [2]. Although themicrowave synthesis of metal nanoparticles has been studied<strong>in</strong> the literature, the microwave process<strong>in</strong>g of the colloids ofmetal nanoparticles has not been <strong>in</strong>vestigated <strong>in</strong> detail. Theevaporation <strong>in</strong>duced self-assembly of zeolite patterns wasreported at room temperature recently [3].Here, the deposition of alum<strong>in</strong>um and silver microwires wasdirected by the evaporation-<strong>in</strong>duced self-assembly ofnanoparticles under the microwave heat<strong>in</strong>g [4]. Compared tothe conventional heat<strong>in</strong>g, the microwave radiation had manyadvantages, such as very short time heat<strong>in</strong>g, homogeneousenergy transfer to the liquid and reduced bubble formation <strong>in</strong>solution. The formation of microwire patterns was due to thestick-slip dynamics of the contact l<strong>in</strong>e on the surface of thesubstrates. By us<strong>in</strong>g the microwave energy, the rapid selfassemblyof the microwires from the metal nanoparticles wasachieved with<strong>in</strong> a few m<strong>in</strong>utes for the first time.The contact l<strong>in</strong>e deposition of nanoparticles has been studiedby several groups to prepare micropatterns of variousmaterials. In this study, the effects of us<strong>in</strong>g microwave heat<strong>in</strong>gwere explored for the first time to accelerate the particledeposition process. As shown <strong>in</strong> Figure 1, the role ofmicrowave dur<strong>in</strong>g the stick-slip motion of contact l<strong>in</strong>e wasFigure 2: The images of nanoalum<strong>in</strong>um and nanosilvermicrowire patterns deposited on glass substrates at 55 C undermicrowave heat<strong>in</strong>g [4].20 °C without heat<strong>in</strong>g, at 40 °C with conventional heat<strong>in</strong>g andat 55 °C with microwave heat<strong>in</strong>g. As demonstrated <strong>in</strong> Figure2, the microwire patterns produced us<strong>in</strong>g the microwaveheat<strong>in</strong>g are very different for nano Al and Ag particles [4].In summary, it was demonstrated that the colloidal selfassemblyof particles under microwave is an efficient methodto produce micropatterns of nanoparticles. The microwiredeposition process presented <strong>in</strong> this study is relatively simplecompare to the previous pattern<strong>in</strong>g techniques. The use ofphotoresist layer, micropatterned mask, monolayer coat<strong>in</strong>gand molded patterns is not required. As a result of thesef<strong>in</strong>d<strong>in</strong>gs, it was concluded that the colloids of alum<strong>in</strong>um andsilver nanoparticles are suitable for the rapid self-assembly ofthe microwire patterns under the microwave heat<strong>in</strong>g.*Correspond<strong>in</strong>g author: HTuolgun@sakarya.edu.trTFigure 1. The mechanism of microwire deposition demonstrated bythe stick-slip dynamics of the contact l<strong>in</strong>e [4].<strong>in</strong>vestigated us<strong>in</strong>g the colloids of nano Al and Ag particles.The colloid solutions of 0.03%(w/v) nanoalum<strong>in</strong>umconta<strong>in</strong><strong>in</strong>g 10%(v/v) PDMS were placed <strong>in</strong> glass vials and thedeposition of microwires on the wall surface was carried out at[1] S.H. Kim, S.Y. Lee, G.R. Yi, D.J. P<strong>in</strong>e, S.M. Yang, J. Am. Chem.Soc. 128, 10897, (2006).[2] J.C. Yu, X.L. Hu, L.B. Quan, L.Z. Zhang, Chem. Commun. 21,2704, (2005).[3] U. Olgun, V. Sev<strong>in</strong>ç, Powder Tech. 183, 207, (2008).[4] U.Olgun. ACS Appl. Mater. Interfaces. 2(1), 28, (2010).6th Nanoscience and Nanotechnology Conference, zmir, 2010 653
- Page 1: Poster Presentations3rd Day17 June
- Page 4 and 5: Determination of Dielectric Anisotr
- Page 7 and 8: Poster Session, Thursday, June 17Th
- Page 9 and 10: PP mPP vs.P =P,PP (1)P andPoster Se
- Page 11 and 12: PP mPP vs.P =P,PP (1)P andPoster Se
- Page 13 and 14: PP andPoster Session, Thursday, Jun
- Page 15 and 16: Poster Session, Thursday, June 17Th
- Page 17 and 18: PP and770 772 774 776 778 780 782 7
- Page 19 and 20: Poster Session, Thursday, June 17Th
- Page 21 and 22: Poster Session, Thursday, June 17Th
- Page 23 and 24: P25,Poster Session, Thursday, June
- Page 25 and 26: PP TOBBPoster Session, Thursday, Ju
- Page 27 and 28: PisPPisisisP,PisPoster Session, Thu
- Page 29 and 30: U NeslihanPPPPoster Session, Thursd
- Page 31 and 32: Poster Session, Thursday, June 17Th
- Page 33 and 34: PPPoster Session, Thursday, June 17
- Page 35 and 36: PPoster Session, Thursday, June 17T
- Page 37 and 38: P onP viaPP wereP upPoster Session,
- Page 39 and 40: P ·cm.PVPPPsPPPPP andPoster Sessio
- Page 41: Poster Session, Thursday, June 17Th
- Page 45 and 46: PPoster Session, Thursday, June 17T
- Page 47 and 48: Poster Session, Thursday, June 17Th
- Page 49 and 50: PErkanPoster Session, Thursday, Jun
- Page 51 and 52: Poster Session, Thursday, June 17Th
- Page 53 and 54: Poster Session, Thursday, June 17Th
- Page 55 and 56: PPPP andPoster Session, Thursday, J
- Page 57 and 58: Poster Session, Thursday, June 17Th
- Page 59 and 60: Poster Session, Thursday, June 17Th
- Page 61 and 62: T PeptideTPP,PP,PP andTT2429TTTTTT
- Page 63 and 64: Poster Session, Thursday, June 17Th
- Page 65 and 66: PPoster Session, Thursday, June 17T
- Page 67 and 68: Poster Session, Thursday, June 17Th
- Page 69 and 70: PPPoster Session, Thursday, June 17
- Page 71 and 72: Poster Session, Thursday, June 17Th
- Page 73 and 74: Poster Session, Thursday, June 17Th
- Page 75 and 76: PT AdditionalT ThePoster Session, T
- Page 77 and 78: Poster Session, Thursday, June 17Th
- Page 79 and 80: Poster Session, Thursday, June 17Th
- Page 81 and 82: Poster Session, Thursday, June 17Th
- Page 83 and 84: PPoster Session, Thursday, June 17T
- Page 85 and 86: Poster Session, Thursday, June 17Th
- Page 87 and 88: PPPoster Session, Thursday, June 17
- Page 89 and 90: Poster Session, Thursday, June 17Hu
- Page 91 and 92: Poster Session, Thursday, June 17Th
- Page 93 and 94:
PPPPPPoster Session, Thursday, June
- Page 95 and 96:
Poster Session, Thursday, June 17Th
- Page 97 and 98:
Poster Session, Thursday, June 17Th
- Page 99 and 100:
Poster Session, Thursday, June 17Th
- Page 101 and 102:
PPoster Session, Thursday, June 17T
- Page 103 and 104:
Poster Session, Thursday, June 17Th
- Page 105 and 106:
PPPPPPPoster Session, Thursday, Jun
- Page 107 and 108:
Poster Session, Thursday, June 17Th
- Page 109 and 110:
PPPR2R PIN(80)PPgPP OzlemPPoster Se
- Page 111 and 112:
Poster Session, Thursday, June 17Th
- Page 113 and 114:
Poster Session, Thursday, June 17Th
- Page 115 and 116:
P onPP toP coordinatedPPoster Sessi
- Page 117 and 118:
PPPPP,PP,P(PR RmPoster Session, Thu
- Page 119 and 120:
Poster Session, Thursday, June 17Th
- Page 121 and 122:
Poster Session, Thursday, June 17Th
- Page 123 and 124:
PP InstitutePP DepartmentPoster Ses
- Page 125 and 126:
andPCPPoster Session, Thursday, Jun
- Page 127 and 128:
PP scatteringPYusufPP Corresponding
- Page 129 and 130:
PP toPoster Session, Thursday, June
- Page 131 and 132:
PP andPoster Session, Thursday, Jun
- Page 133 and 134:
PPPPoster Session, Thursday, June 1
- Page 135 and 136:
PPoster Session, Thursday, June 17T
- Page 137 and 138:
PPP andP (.cm).Poster Session, Thur
- Page 139 and 140:
PP tiltP andP editionPoster Session
- Page 141 and 142:
PP andPPoster Session, Thursday, Ju
- Page 143 and 144:
Poster Session, Thursday, June 17Th
- Page 145 and 146:
PP forP forP edit.PPoster Session,
- Page 147 and 148:
Poster Session, Thursday, June 17Th
- Page 149 and 150:
Poster Session, Thursday, June 17Th
- Page 151 and 152:
PP ionicPP ,PPoster Session, Thursd
- Page 153 and 154:
PP lightPoster Session, Thursday, J
- Page 155 and 156:
Poster Session, Thursday, June 17Th
- Page 157 and 158:
PPoster Session, Thursday, June 17T
- Page 159 and 160:
Poster Session, Thursday, June 17Th
- Page 161 and 162:
PandPoster Session, Thursday, June
- Page 163 and 164:
Poster Session, Thursday, June 17 T
- Page 165 and 166:
PPPoster Session, Thursday, June 17
- Page 167 and 168:
PPoster Session, Thursday, June 17T
- Page 169 and 170:
PPoster Session, Thursday, June 17T
- Page 171 and 172:
PPoster Session, Thursday, June 17T
- Page 173 and 174:
PP DepartmentNanoscienceTPPoster Se
- Page 175 and 176:
Poster Session, Thursday, June 17Th
- Page 177 and 178:
Poster Session, Thursday, June 17Th
- Page 179 and 180:
PPPoster Session, Thursday, June 17
- Page 181 and 182:
PPPPPoster Session, Thursday, June
- Page 183 and 184:
PPPPoster Session, Thursday, June 1
- Page 185 and 186:
PPoster Session, Thursday, June 17T
- Page 187 and 188:
PPoster Session, Thursday, June 17T
- Page 189 and 190:
PPoster Session, Thursday, June 17T
- Page 191 and 192:
Poster Session, Thursday, June 17Th
- Page 193 and 194:
Poster Session, Thursday, June 17Th
- Page 195 and 196:
0T0T0T0T AsPPPP werePoster Session,
- Page 197 and 198:
PPoster Session, Thursday, June 17T
- Page 199 and 200:
PPPPPoster Session, Thursday, June
- Page 201 and 202:
PPoster Session, Thursday, June 17T
- Page 203 and 204:
PPoster Session, Thursday, June 17T
- Page 205 and 206:
Poster Session, Thursday, June 17Th
- Page 207 and 208:
PPoster Session, Thursday, June 17T
- Page 209 and 210:
PPoster Session, Thursday, June 17T
- Page 211:
Poster Session, Thursday, June 17AF