Poster Session, Thursday, June 17Theme F686 - N1123Nanotechnology Applications <strong>in</strong> Food IndustryGonca Susyal 1 * Neriman Badatlolu 11 Department of Food Eng<strong>in</strong>eer<strong>in</strong>g, Celal Bayar University, 45140 Manisa, TurkeyAbstract- In this review, we focus on the advantages and disadvantages of nanotechnology applications that are used <strong>in</strong> food <strong>in</strong>dustry toimprove quality of products and food packag<strong>in</strong>g materials and the development of smart foods. We will also discuss the implications of foodnanotechnology and identify current problem areas <strong>in</strong> nanotechnology <strong>in</strong> view of the potential risks of nanomaterials for health and theenvironment, as well as regulatory issues.Nanotechnology generally refers to objects that are onebillionthof a meter <strong>in</strong> diameter (nanometer). The pr<strong>in</strong>ciple ofnanotechnology is that materials with known properties andfunctions at their normal sizes take on different and oftenuseful properties and functions at their nanosizes [1]. Whenthe reduction <strong>in</strong> size of structures leads to step changes <strong>in</strong>properties, that provide radical new solutions to problems andnew commercial opportunities, these types of applications areconsidered to be examples of what has been termedevolutionary nanotechnology [2].In the food <strong>in</strong>dustry, several novel applications ofnanotechnologies have become apparent, <strong>in</strong>clud<strong>in</strong>g the use ofnanoparticles, such as micelles, liposomes, nanoemulsions,biopolymeric nanoparticles and cubosomes, as well as thedevelopment of nanosensors, which are aimed at ensur<strong>in</strong>gfood safety [2,3]. Also, nanotechnologies cover many aspects,such as disease treatment, food security, new materials forpathogen detection, packag<strong>in</strong>g materials and delivery systems[4].Figure 1. Application matrix of nanotechnology <strong>in</strong> food science andtechnologyAs it applies to the food <strong>in</strong>dustry, nanotechnology <strong>in</strong>volvesus<strong>in</strong>g biological molecules such as sugars or prote<strong>in</strong>s as targetrecognitiongroups for nanostructures that could be used, forexample, as biosensors on foods [1,5,6]. Such biosensorscould serve as detectors of food pathogens and othercontam<strong>in</strong>ants and as devices to track food products [7].Nanotechnology may also be useful <strong>in</strong> encapsulation systemsfor protection aga<strong>in</strong>st environmental factors. In addition, it canbe used <strong>in</strong> the design of food <strong>in</strong>gredients such as flavors andantioxidants [4]. The goal is to improve the functionality ofsuch <strong>in</strong>gredients while m<strong>in</strong>imiz<strong>in</strong>g their concentration. As the<strong>in</strong>fusion of novel <strong>in</strong>gredients <strong>in</strong>to foods ga<strong>in</strong>s popularity,greater exploration of delivery and controlled-release systemsfor nutraceuticals will occur [8].Although nanotechnology can potentially be useful <strong>in</strong> allareas of food production and process<strong>in</strong>g, many of the methodsare either too expensive or too impractical to implement on acommercial scale. For this reason, nanoscale techniques aremost cost-effective <strong>in</strong> the follow<strong>in</strong>g areas of the food <strong>in</strong>dustry:development of new functional materials, food formulations,food process<strong>in</strong>g at microscale and nanoscale levels, productdevelopment, and storage [1,2,7]. Besides, nanotechnologyhas the potential to improve the environment, both throughdirect applications of nano-materials to detect, prevent, andremove pollutants, as well as <strong>in</strong>directly by us<strong>in</strong>gnanotechnology to design cleaner <strong>in</strong>dustrial processes andcreate environmentally responsible products and to providemore sensitive detection systems for air and water qualitymonitor<strong>in</strong>g [10].It is important to note that nanomaterials, ow<strong>in</strong>g to their<strong>in</strong>creased contact surface area, might have toxic effects <strong>in</strong> thebody that are not apparent <strong>in</strong> the bulk materials. In addition,there might be potential and unforeseen risks for their use <strong>in</strong>food-packag<strong>in</strong>g materials [3]. While nanotechnology mightprovide solutions for certa<strong>in</strong> environmental problems,relatively little is known at present about the environmentalimpact of nano-particles. Current studies <strong>in</strong>dicate that somenanomaterials are toxic and they can impact biodegradation,transformation and adsorption of some other contam<strong>in</strong>ants <strong>in</strong>the environment [10].However, there are social and ethical issues of us<strong>in</strong>gnanotechnology <strong>in</strong> the food sector that must be considered.Currently, the potential risks of nanomaterials to human healthand to the environment are unknown. Governments shouldconsider appropriate label<strong>in</strong>g and should also set downregulations that will help to <strong>in</strong>crease consumer acceptability[3]. At this stage of (lack of) knowledge of nanotoxicology itis unavoidable that risk assessors need as much <strong>in</strong>formation aspossible about nanoparticals and their appearance andbehavior <strong>in</strong> biological matrices and organisms [4].*Correspond<strong>in</strong>g author: 0Hgoncasusyal@gmail.com[1]Richardson, S.M.N., Journal of the American Dietetic Association,2007, 1494-1497.[2] Chau,C.F., Wu,S.H., G.C.,Yen, Trends <strong>in</strong> Food Science &Technology,18, 2007, 269-280.[3] Sozer,N., Kok<strong>in</strong>i,J.L., 2009, Trends <strong>in</strong> Biotechnology,27,2, 82-89.[4] H. Bouwmeester et al. / Regulatory Toxicology andPharmacology 53 (2009) 52–62[5] Baeummer, A. (2004), Food Technol. 58, 51–55[6] Vo-D<strong>in</strong>h, T. et al. (2001), Sensors Actuat. B. 74, 2–11[7] Azeredo,H.M.C., Food Research International 42 (2009) 1240–1253[8] Haruyama, T. 2003, Adv. Drug Delivery Rev. 55: 393-401.[9] Kaplan,.., Karanfil,T., Kiti,M., 7. Ulusal Çerce MühendisliiKongresi, 2007, 845-848.6th Nanoscience and Nanotechnology Conference, zmir, 2010 773
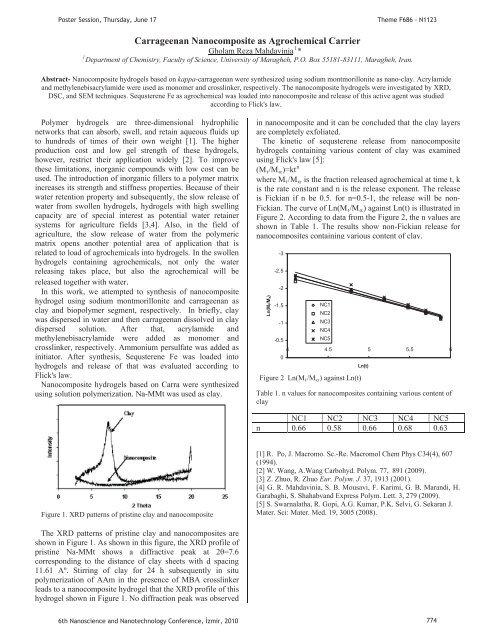
PPoster Session, Thursday, June 17Theme F686 - N11231Carrageenan Nanocomposite as Agrochemical Carrier1UGholam Reza Mahdav<strong>in</strong>iaUP P*PDepartment of Chemistry, Faculty of Science, University of Maragheh, P.O. Box 55181-83111, Maragheh, Iran.Abstract- Nanocomposite hydrogels based on kappa-carrageenan were synthesized us<strong>in</strong>g sodium montmorillonite as nano-clay. Acrylamideand methylenebisacrylamide were used as monomer and crossl<strong>in</strong>ker, respectively. The nanocomposite hydrogels were <strong>in</strong>vestigated by XRD,DSC, and SEM techniques. Sequsterene Fe as agrochemical was loaded <strong>in</strong>to nanocomposite and release of this active agent was studiedaccord<strong>in</strong>g to Flick's law.Polymer hydrogels are three-dimensional hydrophilicnetworks that can absorb, swell, and reta<strong>in</strong> aqueous fluids upto hundreds of times of their own weight [1]. The higherproduction cost and low gel strength of these hydrogels,however, restrict their application widely [2]. To improvethese limitations, <strong>in</strong>organic compounds with low cost can beused. The <strong>in</strong>troduction of <strong>in</strong>organic fillers to a polymer matrix<strong>in</strong>creases its strength and stiffness properties. Because of theirwater retention property and subsequently, the slow release ofwater from swollen hydrogels, hydrogels with high swell<strong>in</strong>gcapacity are of special <strong>in</strong>terest as potential water reta<strong>in</strong>ersystems for agriculture fields [3,4]. Also, <strong>in</strong> the field ofagriculture, the slow release of water from the polymericmatrix opens another potential area of application that isrelated to load of agrochemicals <strong>in</strong>to hydrogels. In the swollenhydrogels conta<strong>in</strong><strong>in</strong>g agrochemicals, not only the waterreleas<strong>in</strong>g takes place, but also the agrochemical will bereleased together with water.In this work, we attempted to synthesis of nanocompositehydrogel us<strong>in</strong>g sodium montmorillonite and carrageenan asclay and biopolymer segment, respectively. In briefly, claywas dispersed <strong>in</strong> water and then carrageenan dissolved <strong>in</strong> claydispersed solution. After that, acrylamide andmethylenebisacrylamide were added as monomer andcrossl<strong>in</strong>ker, respectively. Ammonium persulfate was added as<strong>in</strong>itiator. After synthesis, Sequsterene Fe was loaded <strong>in</strong>tohydrogels and release of that was evaluated accord<strong>in</strong>g toFlick's law.Nanocomposite hydrogels based on Carra were synthesizedus<strong>in</strong>g solution polymerization. Na-MMt was used as clay.<strong>in</strong> nanocomposite and it can be concluded that the clay layersare completely exfoliated.The k<strong>in</strong>etic of sequsterene release from nanocompositehydrogels conta<strong>in</strong><strong>in</strong>g various content of clay was exam<strong>in</strong>edus<strong>in</strong>g Flick's law [5]:n(MRtR/MRR)=ktPwhere MRtR/MRR is the fraction released agrochemical at time t, kis the rate constant and n is the release exponent. The releaseis Fickian if n be 0.5. for n=0.5-1, the release will be non-Fickian. The curve of Ln(MRtR/MRR) aga<strong>in</strong>st Ln(t) is illustrated <strong>in</strong>Figure 2. Accord<strong>in</strong>g to data from the Figure 2, the n values areshown <strong>in</strong> Table 1. The results show non-Fickian release fornanocomposites conta<strong>in</strong><strong>in</strong>g various content of clay.Ln(Mt/Mx)-3-2.5-2-1.5-1-0.50NC1NC2NC3NC4NC54 4.5 5 5.5 6Figure 2 Ln(MRtR/MRR) aga<strong>in</strong>st Ln(t)Ln(t)Table 1. n values for nanocomposites conta<strong>in</strong><strong>in</strong>g various content ofclayNC1 NC2 NC3 NC4 NC5n 0.66 0.58 0.66 0.68 0.63Figure 1. XRD patterns of prist<strong>in</strong>e clay and nanocomposite[1] R. Po, J. Macromo. Sc.-Re. Macromol Chem Phys C34(4), 607(1994).[2] W. Wang, A.Wang Carbohyd. Polym. 77, 891 (2009).[3] Z. Zhuo, R. Zhuo Eur. Polym. J. 37, 1913 (2001).[4] G. R. Mahdav<strong>in</strong>ia, S. B. Mousavi, F. Karimi, G. B. Marandi, H.Garabaghi, S. Shahabvand Express Polym. Lett. 3, 279 (2009).[5] S. Swarnalatha, R. Gopi, A.G. Kumar, P.K. Selvi, G. Sekaran J.Mater. Sci: Mater. Med. 19, 3005 (2008).The XRD patterns of prist<strong>in</strong>e clay and nanocomposites areshown <strong>in</strong> Figure 1. As shown <strong>in</strong> this figure, the XRD profile ofprist<strong>in</strong>e Na-MMt shows a diffractive peak at 2=7.6correspond<strong>in</strong>g to the distance of clay sheets with d spac<strong>in</strong>g11.61 Aº. Stirr<strong>in</strong>g of clay for 24 h subsequently <strong>in</strong> situpolymerization of AAm <strong>in</strong> the presence of MBA crossl<strong>in</strong>kerleads to a nanocomposite hydrogel that the XRD profile of thishydrogel shown <strong>in</strong> Figure 1. No diffraction peak was observed6th Nanoscience and Nanotechnology Conference, zmir, 2010 774
- Page 1:
Poster Presentations3rd Day17 June
- Page 4 and 5:
Determination of Dielectric Anisotr
- Page 7 and 8:
Poster Session, Thursday, June 17Th
- Page 9 and 10:
PP mPP vs.P =P,PP (1)P andPoster Se
- Page 11 and 12:
PP mPP vs.P =P,PP (1)P andPoster Se
- Page 13 and 14:
PP andPoster Session, Thursday, Jun
- Page 15 and 16:
Poster Session, Thursday, June 17Th
- Page 17 and 18:
PP and770 772 774 776 778 780 782 7
- Page 19 and 20:
Poster Session, Thursday, June 17Th
- Page 21 and 22:
Poster Session, Thursday, June 17Th
- Page 23 and 24:
P25,Poster Session, Thursday, June
- Page 25 and 26:
PP TOBBPoster Session, Thursday, Ju
- Page 27 and 28:
PisPPisisisP,PisPoster Session, Thu
- Page 29 and 30:
U NeslihanPPPPoster Session, Thursd
- Page 31 and 32:
Poster Session, Thursday, June 17Th
- Page 33 and 34:
PPPoster Session, Thursday, June 17
- Page 35 and 36:
PPoster Session, Thursday, June 17T
- Page 37 and 38:
P onP viaPP wereP upPoster Session,
- Page 39 and 40:
P ·cm.PVPPPsPPPPP andPoster Sessio
- Page 41 and 42:
Poster Session, Thursday, June 17Th
- Page 43 and 44:
PPoster Session, Thursday, June 17T
- Page 45 and 46:
PPoster Session, Thursday, June 17T
- Page 47 and 48:
Poster Session, Thursday, June 17Th
- Page 49 and 50:
PErkanPoster Session, Thursday, Jun
- Page 51 and 52:
Poster Session, Thursday, June 17Th
- Page 53 and 54:
Poster Session, Thursday, June 17Th
- Page 55 and 56:
PPPP andPoster Session, Thursday, J
- Page 57 and 58:
Poster Session, Thursday, June 17Th
- Page 59 and 60:
Poster Session, Thursday, June 17Th
- Page 61 and 62:
T PeptideTPP,PP,PP andTT2429TTTTTT
- Page 63 and 64:
Poster Session, Thursday, June 17Th
- Page 65 and 66:
PPoster Session, Thursday, June 17T
- Page 67 and 68:
Poster Session, Thursday, June 17Th
- Page 69 and 70:
PPPoster Session, Thursday, June 17
- Page 71 and 72:
Poster Session, Thursday, June 17Th
- Page 73 and 74:
Poster Session, Thursday, June 17Th
- Page 75 and 76:
PT AdditionalT ThePoster Session, T
- Page 77 and 78:
Poster Session, Thursday, June 17Th
- Page 79 and 80:
Poster Session, Thursday, June 17Th
- Page 81 and 82:
Poster Session, Thursday, June 17Th
- Page 83 and 84:
PPoster Session, Thursday, June 17T
- Page 85 and 86:
Poster Session, Thursday, June 17Th
- Page 87 and 88:
PPPoster Session, Thursday, June 17
- Page 89 and 90:
Poster Session, Thursday, June 17Hu
- Page 91 and 92:
Poster Session, Thursday, June 17Th
- Page 93 and 94:
PPPPPPoster Session, Thursday, June
- Page 95 and 96:
Poster Session, Thursday, June 17Th
- Page 97 and 98:
Poster Session, Thursday, June 17Th
- Page 99 and 100:
Poster Session, Thursday, June 17Th
- Page 101 and 102:
PPoster Session, Thursday, June 17T
- Page 103 and 104:
Poster Session, Thursday, June 17Th
- Page 105 and 106:
PPPPPPPoster Session, Thursday, Jun
- Page 107 and 108:
Poster Session, Thursday, June 17Th
- Page 109 and 110:
PPPR2R PIN(80)PPgPP OzlemPPoster Se
- Page 111 and 112:
Poster Session, Thursday, June 17Th
- Page 113 and 114:
Poster Session, Thursday, June 17Th
- Page 115 and 116: P onPP toP coordinatedPPoster Sessi
- Page 117 and 118: PPPPP,PP,P(PR RmPoster Session, Thu
- Page 119 and 120: Poster Session, Thursday, June 17Th
- Page 121 and 122: Poster Session, Thursday, June 17Th
- Page 123 and 124: PP InstitutePP DepartmentPoster Ses
- Page 125 and 126: andPCPPoster Session, Thursday, Jun
- Page 127 and 128: PP scatteringPYusufPP Corresponding
- Page 129 and 130: PP toPoster Session, Thursday, June
- Page 131 and 132: PP andPoster Session, Thursday, Jun
- Page 133 and 134: PPPPoster Session, Thursday, June 1
- Page 135 and 136: PPoster Session, Thursday, June 17T
- Page 137 and 138: PPP andP (.cm).Poster Session, Thur
- Page 139 and 140: PP tiltP andP editionPoster Session
- Page 141 and 142: PP andPPoster Session, Thursday, Ju
- Page 143 and 144: Poster Session, Thursday, June 17Th
- Page 145 and 146: PP forP forP edit.PPoster Session,
- Page 147 and 148: Poster Session, Thursday, June 17Th
- Page 149 and 150: Poster Session, Thursday, June 17Th
- Page 151 and 152: PP ionicPP ,PPoster Session, Thursd
- Page 153 and 154: PP lightPoster Session, Thursday, J
- Page 155 and 156: Poster Session, Thursday, June 17Th
- Page 157 and 158: PPoster Session, Thursday, June 17T
- Page 159 and 160: Poster Session, Thursday, June 17Th
- Page 161 and 162: PandPoster Session, Thursday, June
- Page 163 and 164: Poster Session, Thursday, June 17 T
- Page 165: PPPoster Session, Thursday, June 17
- Page 169 and 170: PPoster Session, Thursday, June 17T
- Page 171 and 172: PPoster Session, Thursday, June 17T
- Page 173 and 174: PP DepartmentNanoscienceTPPoster Se
- Page 175 and 176: Poster Session, Thursday, June 17Th
- Page 177 and 178: Poster Session, Thursday, June 17Th
- Page 179 and 180: PPPoster Session, Thursday, June 17
- Page 181 and 182: PPPPPoster Session, Thursday, June
- Page 183 and 184: PPPPoster Session, Thursday, June 1
- Page 185 and 186: PPoster Session, Thursday, June 17T
- Page 187 and 188: PPoster Session, Thursday, June 17T
- Page 189 and 190: PPoster Session, Thursday, June 17T
- Page 191 and 192: Poster Session, Thursday, June 17Th
- Page 193 and 194: Poster Session, Thursday, June 17Th
- Page 195 and 196: 0T0T0T0T AsPPPP werePoster Session,
- Page 197 and 198: PPoster Session, Thursday, June 17T
- Page 199 and 200: PPPPPoster Session, Thursday, June
- Page 201 and 202: PPoster Session, Thursday, June 17T
- Page 203 and 204: PPoster Session, Thursday, June 17T
- Page 205 and 206: Poster Session, Thursday, June 17Th
- Page 207 and 208: PPoster Session, Thursday, June 17T
- Page 209 and 210: PPoster Session, Thursday, June 17T
- Page 211: Poster Session, Thursday, June 17AF