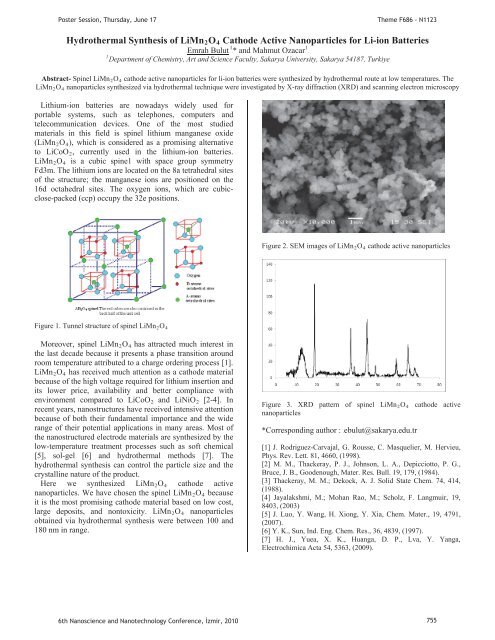
PPPoster Session, Thursday, June 17Theme F686 - N1123Hydrothermal Synthesis of LiMnR2ROR4R Cathode Active Nanoparticles for Li-ion Batteries11UEmrah BulutUP P* and Mahmut OzacarP1PDepartment of Chemistry, Art and Science Faculty, Sakarya University, Sakarya 54187, TurkiyeAbstract- Sp<strong>in</strong>el LiMnR2ROR4R cathode active nanoparticles for li-ion batteries were synthesized by hydrothermal route at low temperatures. TheLiMnR2ROR4R nanoparticles synthesized via hydrothermal technique were <strong>in</strong>vestigated by X-ray diffraction (XRD) and scann<strong>in</strong>g electron microscopyLithium-ion batteries are nowadays widely used forportable systems, such as telephones, computers andtelecommunication devices. One of the most studiedmaterials <strong>in</strong> this field is sp<strong>in</strong>el lithium manganese oxide(LiMnR2ROR4R), which is considered as a promis<strong>in</strong>g alternativeto LiCoOR2R, currently used <strong>in</strong> the lithium-ion batteries.LiMnR2ROR4R is a cubic sp<strong>in</strong>e1 with space group symmetryFd3m. The lithium ions are located on the 8a tetrahedral sitesof the structure; the manganese ions are positioned on the16d octahedral sites. The oxygen ions, which are cubicclose-packed(ccp) occupy the 32e positions.Figure 2. SEM images of LiMnR2ROR4R cathode active nanoparticlesFigure 1. Tunnel structure of sp<strong>in</strong>el LiMnR2ROR4RMoreover, sp<strong>in</strong>el LiMnR2ROR4R has attracted much <strong>in</strong>terest <strong>in</strong>the last decade because it presents a phase transition aroundroom temperature attributed to a charge order<strong>in</strong>g process [1].LiMnR2ROR4R has received much attention as a cathode materialbecause of the high voltage required for lithium <strong>in</strong>sertion andits lower price, availability and better compliance withenvironment compared to LiCoOR2R and LiNiOR2R [2-4]. Inrecent years, nanostructures have received <strong>in</strong>tensive attentionbecause of both their fundamental importance and the widerange of their potential applications <strong>in</strong> many areas. Most ofthe nanostructured electrode materials are synthesized by thelow-temperature treatment processes such as soft chemical[5], sol-gel [6] and hydrothermal methods [7]. Thehydrothermal synthesis can control the particle size and thecrystall<strong>in</strong>e nature of the product.Here we synthesized LiMnR2ROR4R cathode activenanoparticles. We have chosen the sp<strong>in</strong>el LiMn R2ROR4R becauseit is the most promis<strong>in</strong>g cathode material based on low cost,large deposits, and nontoxicity. LiMnR2ROR4R nanoparticlesobta<strong>in</strong>ed via hydrothermal synthesis were between 100 and180 nm <strong>in</strong> range.Figure 3. XRD pattern of sp<strong>in</strong>el LiMnR2ROR4R cathode activenanoparticles*Correspond<strong>in</strong>g author : HTebulut@sakarya.edu.trT[1] J. Rodriguez-Carvajal, G. Rousse, C. Masquelier, M. Hervieu,Phys. Rev. Lett. 81, 4660, (1998).[2] M. M., Thackeray, P. J., Johnson, L. A., Depicciotto, P. G.,Bruce, J. B., Goodenough, Mater. Res. Bull. 19, 179, (1984).[3] Thackeray, M. M.; Dekock, A. J. Solid State Chem. 74, 414,(1988).[4] Jayalakshmi, M.; Mohan Rao, M.; Scholz, F. Langmuir, 19,8403, (2003)[5] J. Luo, Y. Wang, H. Xiong, Y. Xia, Chem. Mater., 19, 4791,(2007).[6] Y. K., Sun, Ind. Eng. Chem. Res., 36, 4839, (1997).[7] H. J., Yuea, X. K., Huanga, D. P., Lva, Y. Yanga,Electrochimica Acta 54, 5363, (2009).6th Nanoscience and Nanotechnology Conference, zmir, 2010 755
Poster Session, Thursday, June 17Theme F686 - N1123Preparation of multi-layered Pt/Co cathodes for proton exchange membrane fuel cells (PEM) bydc- magnetron sputter<strong>in</strong>gOguz Kaan Ozdemir 1,2 , Ali Sems Ahsen 2 , Osman Ozturk 2 , Evel<strong>in</strong>a Slavcheva 31 Yildiz Tech Univ, Dept Met & Mat Engn, Istanbul, Turkey2 Nanotechnology Research Canter, Gebze Institute of Technology, Kocaeli, Turkey3 Institute of Electrochemistry and Energy Systems-Bulgarian Academy of Sciences, Sofia, BulgariaAbstract- In order to <strong>in</strong>vestigate the effect of Co layers <strong>in</strong> the cathode electrode a series of unalloyed multilayer Pt/Co th<strong>in</strong> films weredeposited by dc magnetron sputter<strong>in</strong>g upon a th<strong>in</strong> Ti sublayer sputtered on the top of a conductive micro porous carbon diffusion layer.Proton exchange membrane (PEM) fuel cells arepromis<strong>in</strong>g power source due to their good energyconversion efficiency and high power density of their fuelsources [1]. Nevertheless, the achieved substantialprogresses <strong>in</strong> the PEM fuel cells are not broadly utilizeddue to their cost and durability. Precious Pt catalyst is themost important cost factor <strong>in</strong> the PEM fuel cells. Therefore,many researches are focus<strong>in</strong>g on the development ofcompact unit and reduc<strong>in</strong>g the loads on the catalysts [2].The Th<strong>in</strong> film deposition method of magnetron sputter<strong>in</strong>g(MS), which is widely used for <strong>in</strong>tegrated circuitmanufactur<strong>in</strong>g, recently f<strong>in</strong>ds application as an alternativecatalyst preparation and electrode assembl<strong>in</strong>g technique.This method allows deposition of th<strong>in</strong> compact films upon aselected substrate material such as either gas diffusion layeror Nafion, and ensures simplicity of the catalystspreparation as well as improved stability, durability, andutilization [3-5].In our study, a series of unalloyed multilayer Pt/Co th<strong>in</strong>films were deposited by dc magnetron sputter<strong>in</strong>g upon ath<strong>in</strong> Ti sublayer sputtered on the top of a conductive microporous carbon diffusion layer. In order to <strong>in</strong>vestigate theeffect of Co on the oxygen reduction reaction, differentcompositions (70:30, 50:50, 30:70 Pt/Co atomic ratio) wereemployed, while the amount of Pt was constant(21 μg.cm -2 ). Each electrode was <strong>in</strong>vestigated us<strong>in</strong>g theconventional electrochemical methods of cyclicvoltammetry and steady state polarization curves <strong>in</strong> 0.5MH 2 SO 4 as well as a membrane electrode assembly, MEA,cathode <strong>in</strong> a s<strong>in</strong>gle hydrogen PEM fuel cell. The cyclicvoltammograms, CV, were used to calculatethe electrochemically active surface area, EASA, of theelectrode under study, apply<strong>in</strong>g the well establishedprocedure of <strong>in</strong>tegration the area under the hydrogen adsorption / desorption peaks and us<strong>in</strong>g the value of 210mC.cm -2 (the charge required for adsorption of hydrogenmonolayer on 1 cm 2 of smooth Pt electrode) as a correctionfactor [6].The electrocatalytic activity of Pt/Co films toward theoxygen reduction was assessed apply<strong>in</strong>g the method ofl<strong>in</strong>ear sweep voltammetry, LSV, and Koutecky–Levichplots. The rotation disc electrode, RDE, polarization curvesshow characteristic behavior reported <strong>in</strong> the literature forOxygen Reduction reaction, ORR, on Pt <strong>in</strong> acid solutionswith a well dist<strong>in</strong>guished region of k<strong>in</strong>etic mixed, anddiffusion limited reaction rate. Exchange current density, j o ,is known to be a qualitative measure for the <strong>in</strong>tr<strong>in</strong>sicactivity of the catalyst, and its calculation has beenexpla<strong>in</strong>ed elaborately <strong>in</strong> our previous study [7].Table 1. EASA and K<strong>in</strong>etic parameters.SampleName(Pt/Co)EASA(m 2 .gr -1 )b(V.dec -1 )j oap(A.cm -2 )jo(A.cm -2 )70:30 28,789 -0,192 0,00426 1,48E-0850:50 51,826 -0,181 0,00338 6,53E-0930:70 52,461 -0,168 0,00557 1,06E-08As show <strong>in</strong> Table 1, 30:70 Pt/Co atomic ratios has thehighest EASA. Moreover, its apparent exchange currentdensity is higher than other two samples, too. Figure 1shows the polarization curves of a series of MEAs withdifferent Pt/Co atomic ratios.E (V)10,80,60,40,270:3050:5030:700 200 400 600 800 1000J (mA.cm -2 )As shown <strong>in</strong> Figure 1, among the three MEAs coatedwith 70:30, 50:50, 30:70 cathode catalyst layer obta<strong>in</strong>ed bysputter-deposition, consistent with the CV and RDEanalysis, the coated MEA with 30:70 Pt/Co atomic ratiodemonstrates the best cell performance. The polarizationcurve shows a high current density of 974 mA.cm -2 at 0.4V. Microstructure and electrochemical studies <strong>in</strong>dicatedthat the additional Co layers sputter-deposited <strong>in</strong> cathodeelectrode might change the microstructure of the electrodemembrane<strong>in</strong>terface as well as vary charge transfer andmass transport properties of MEAs [8].This research has been carried out <strong>in</strong> the frame of theproject EVRENA-108M139.*Correspond<strong>in</strong>g author: 0Hoguz_kozdemir@hotmail.com[1] R. O’Hayre at al., Journal of Power Sources 109, 483-493,(2003).[2] C.L. Chang et al., Surface & Coat<strong>in</strong>gs Technology, 201, 4442-4446, (2006).[3] W. Zhen-Bo at al., Int J Hydrogen Energy, 34, 4387-94,(2009).[4] H. Andrew at al., J Electrochem Soc, 149, A280-7, (2002).[5] H. Kuo-L<strong>in</strong> at al., J Power Sources, 156, 224-31, (2006).[6] Bard AJ., Faulkner L., 2001. In: Electrochemical methods:fundamentals and applications, (p. L849–57) , vol. 341. NewYork: Wiley.[7] O. Ozturt at al., International Journal of Hydrogen Energy, InPress, (2010).[8] Z. Tang at al., J Appl Electrochem, 39, 1821-1826, (2009).6th Nanoscience and Nanotechnology Conference, zmir, 2010 756
- Page 1:
Poster Presentations3rd Day17 June
- Page 4 and 5:
Determination of Dielectric Anisotr
- Page 7 and 8:
Poster Session, Thursday, June 17Th
- Page 9 and 10:
PP mPP vs.P =P,PP (1)P andPoster Se
- Page 11 and 12:
PP mPP vs.P =P,PP (1)P andPoster Se
- Page 13 and 14:
PP andPoster Session, Thursday, Jun
- Page 15 and 16:
Poster Session, Thursday, June 17Th
- Page 17 and 18:
PP and770 772 774 776 778 780 782 7
- Page 19 and 20:
Poster Session, Thursday, June 17Th
- Page 21 and 22:
Poster Session, Thursday, June 17Th
- Page 23 and 24:
P25,Poster Session, Thursday, June
- Page 25 and 26:
PP TOBBPoster Session, Thursday, Ju
- Page 27 and 28:
PisPPisisisP,PisPoster Session, Thu
- Page 29 and 30:
U NeslihanPPPPoster Session, Thursd
- Page 31 and 32:
Poster Session, Thursday, June 17Th
- Page 33 and 34:
PPPoster Session, Thursday, June 17
- Page 35 and 36:
PPoster Session, Thursday, June 17T
- Page 37 and 38:
P onP viaPP wereP upPoster Session,
- Page 39 and 40:
P ·cm.PVPPPsPPPPP andPoster Sessio
- Page 41 and 42:
Poster Session, Thursday, June 17Th
- Page 43 and 44:
PPoster Session, Thursday, June 17T
- Page 45 and 46:
PPoster Session, Thursday, June 17T
- Page 47 and 48:
Poster Session, Thursday, June 17Th
- Page 49 and 50:
PErkanPoster Session, Thursday, Jun
- Page 51 and 52:
Poster Session, Thursday, June 17Th
- Page 53 and 54:
Poster Session, Thursday, June 17Th
- Page 55 and 56:
PPPP andPoster Session, Thursday, J
- Page 57 and 58:
Poster Session, Thursday, June 17Th
- Page 59 and 60:
Poster Session, Thursday, June 17Th
- Page 61 and 62:
T PeptideTPP,PP,PP andTT2429TTTTTT
- Page 63 and 64:
Poster Session, Thursday, June 17Th
- Page 65 and 66:
PPoster Session, Thursday, June 17T
- Page 67 and 68:
Poster Session, Thursday, June 17Th
- Page 69 and 70:
PPPoster Session, Thursday, June 17
- Page 71 and 72:
Poster Session, Thursday, June 17Th
- Page 73 and 74:
Poster Session, Thursday, June 17Th
- Page 75 and 76:
PT AdditionalT ThePoster Session, T
- Page 77 and 78:
Poster Session, Thursday, June 17Th
- Page 79 and 80:
Poster Session, Thursday, June 17Th
- Page 81 and 82:
Poster Session, Thursday, June 17Th
- Page 83 and 84:
PPoster Session, Thursday, June 17T
- Page 85 and 86:
Poster Session, Thursday, June 17Th
- Page 87 and 88:
PPPoster Session, Thursday, June 17
- Page 89 and 90:
Poster Session, Thursday, June 17Hu
- Page 91 and 92:
Poster Session, Thursday, June 17Th
- Page 93 and 94:
PPPPPPoster Session, Thursday, June
- Page 95 and 96: Poster Session, Thursday, June 17Th
- Page 97 and 98: Poster Session, Thursday, June 17Th
- Page 99 and 100: Poster Session, Thursday, June 17Th
- Page 101 and 102: PPoster Session, Thursday, June 17T
- Page 103 and 104: Poster Session, Thursday, June 17Th
- Page 105 and 106: PPPPPPPoster Session, Thursday, Jun
- Page 107 and 108: Poster Session, Thursday, June 17Th
- Page 109 and 110: PPPR2R PIN(80)PPgPP OzlemPPoster Se
- Page 111 and 112: Poster Session, Thursday, June 17Th
- Page 113 and 114: Poster Session, Thursday, June 17Th
- Page 115 and 116: P onPP toP coordinatedPPoster Sessi
- Page 117 and 118: PPPPP,PP,P(PR RmPoster Session, Thu
- Page 119 and 120: Poster Session, Thursday, June 17Th
- Page 121 and 122: Poster Session, Thursday, June 17Th
- Page 123 and 124: PP InstitutePP DepartmentPoster Ses
- Page 125 and 126: andPCPPoster Session, Thursday, Jun
- Page 127 and 128: PP scatteringPYusufPP Corresponding
- Page 129 and 130: PP toPoster Session, Thursday, June
- Page 131 and 132: PP andPoster Session, Thursday, Jun
- Page 133 and 134: PPPPoster Session, Thursday, June 1
- Page 135 and 136: PPoster Session, Thursday, June 17T
- Page 137 and 138: PPP andP (.cm).Poster Session, Thur
- Page 139 and 140: PP tiltP andP editionPoster Session
- Page 141 and 142: PP andPPoster Session, Thursday, Ju
- Page 143 and 144: Poster Session, Thursday, June 17Th
- Page 145: PP forP forP edit.PPoster Session,
- Page 149 and 150: Poster Session, Thursday, June 17Th
- Page 151 and 152: PP ionicPP ,PPoster Session, Thursd
- Page 153 and 154: PP lightPoster Session, Thursday, J
- Page 155 and 156: Poster Session, Thursday, June 17Th
- Page 157 and 158: PPoster Session, Thursday, June 17T
- Page 159 and 160: Poster Session, Thursday, June 17Th
- Page 161 and 162: PandPoster Session, Thursday, June
- Page 163 and 164: Poster Session, Thursday, June 17 T
- Page 165 and 166: PPPoster Session, Thursday, June 17
- Page 167 and 168: PPoster Session, Thursday, June 17T
- Page 169 and 170: PPoster Session, Thursday, June 17T
- Page 171 and 172: PPoster Session, Thursday, June 17T
- Page 173 and 174: PP DepartmentNanoscienceTPPoster Se
- Page 175 and 176: Poster Session, Thursday, June 17Th
- Page 177 and 178: Poster Session, Thursday, June 17Th
- Page 179 and 180: PPPoster Session, Thursday, June 17
- Page 181 and 182: PPPPPoster Session, Thursday, June
- Page 183 and 184: PPPPoster Session, Thursday, June 1
- Page 185 and 186: PPoster Session, Thursday, June 17T
- Page 187 and 188: PPoster Session, Thursday, June 17T
- Page 189 and 190: PPoster Session, Thursday, June 17T
- Page 191 and 192: Poster Session, Thursday, June 17Th
- Page 193 and 194: Poster Session, Thursday, June 17Th
- Page 195 and 196: 0T0T0T0T AsPPPP werePoster Session,
- Page 197 and 198:
PPoster Session, Thursday, June 17T
- Page 199 and 200:
PPPPPoster Session, Thursday, June
- Page 201 and 202:
PPoster Session, Thursday, June 17T
- Page 203 and 204:
PPoster Session, Thursday, June 17T
- Page 205 and 206:
Poster Session, Thursday, June 17Th
- Page 207 and 208:
PPoster Session, Thursday, June 17T
- Page 209 and 210:
PPoster Session, Thursday, June 17T
- Page 211:
Poster Session, Thursday, June 17AF