Poster Session, Thursday, June 17Theme F686 - N1123Direct measurement of humidity adsorption k<strong>in</strong>etics of Calix[4]arene derivative us<strong>in</strong>g QCM techniqueOmer Mermer 1* , Salih Okur 2 , Fevzi Sümer 1 , Mahmut Ku 3 , eref Ertul 4 , Mevlüt Bayrakç 4 , Mustafa Ylmaz 41 Ege University, Department of Electrical and Electronics Eng<strong>in</strong>eer<strong>in</strong>g Bornova/Izmir/TURKEY2 Izmir Institute of Technology, Department of Physics Urla/Izmir/TURKEY3 Selçuk University, Department of Chemical Eng<strong>in</strong>eer<strong>in</strong>g, Selçuklu/Konya/TURKEY4 Selçuk University, Department of Chemistry, Selçuklu/Konya/TURKEYAbstract— This study focuses on the optimization and characterization of calix[4]arene derivative based sensor film coated ona quartz substrate by drop cast<strong>in</strong>g method for use <strong>in</strong> the detection of humidity. The humidity adsorption and desorption k<strong>in</strong>eticscalix[4]arene were <strong>in</strong>vestigated by Quartz Crystal Microbalance (QCM) technique. The Langmuir model was used todeterm<strong>in</strong>e the k<strong>in</strong>etic parameters such as adsorption, desorption rates and Gibbs free energy between relative humidity between11% and 97%. Our reproducible experimental results show that calix[4]arene films have a great potential for humidity sens<strong>in</strong>gapplications at room temperature operations.Monitor<strong>in</strong>g and control of humidity is essential for<strong>in</strong>dustrial progress of the world such as petroleum <strong>in</strong>dustry,medical equipments, food <strong>in</strong>dustry and the manufacturer ofmoisture sensitive products. Furthermore clean rooms,greenhouses, research and developments labs are allenvironments that are highly effected by moisture levels andrequire constant monitor<strong>in</strong>g [1–2].Quartz crystal microbalances (QCMs) have been widelyused <strong>in</strong> recently as promis<strong>in</strong>g gas sensor applications ow<strong>in</strong>g totheir high sensitivity and ease of measurement, s<strong>in</strong>ce themeasured frequency shift is directly proportional to the masschange on a quartz crystal [1–2].Th<strong>in</strong> films of calix[4]arene derivatives have been widelyused <strong>in</strong> chemical sensors. Due to their zeolite-like capacityand selectivity, calix[4]arene became promis<strong>in</strong>g materials forsensor applications. The functional groups at the upper andlower rims determ<strong>in</strong>e their selectivity <strong>in</strong> host-guest<strong>in</strong>teractions and physical properties [3]. Calix[4]arenederivatives have been used <strong>in</strong> recent times as gas sensorsapplications [4,5].The frequency response curve QCM-based sensor tocyclic humidity change is depicted <strong>in</strong> Figure 2 for differentRH levels. The frequency shift of QCM decreases sharplywith <strong>in</strong>creas<strong>in</strong>g humidity concentrations while there is nochange <strong>in</strong> that of the empty QCM (adsorption process). On theother hand, dur<strong>in</strong>g the desorption process, the humidity levelis turned back to the <strong>in</strong>itial value, as a result, QCM recoversback to its <strong>in</strong>itial resonance frequency value.Langmuir adsorption isotherm model is frequentlyused to describe adsorption and desorption k<strong>in</strong>etics of gasvapor molecules onto organic or <strong>in</strong>organic films [6-7].Accord<strong>in</strong>g to this model, the rate of surface reaction forform<strong>in</strong>g a monolayer on the surface is related to fractionalcoverage (), the humidity concentration, and the rateconstants for the adsorption (k a ) and desorption (k d ) processes.Figure 3 shows the experimental data and the fitt<strong>in</strong>g curve forfirst adsorption cycle. k a and k d fitt<strong>in</strong>g parameters determ<strong>in</strong>edfrom the fit us<strong>in</strong>g Langmuir equation to the experimental dataare given by 62.49 M -1 s -1 and 0.0005 s -1 , respectively [8].108Figure1 Chemical formula and full name of special designcalix[4]arene.In this work, special design calix[4]arene molecules weredesigned and synthesized for <strong>in</strong>creas<strong>in</strong>g moisture captur<strong>in</strong>gfeature. Chemical structure and full name of this molecule isgiven <strong>in</strong> Figure 1. We have used QCM technique for humiditydetection us<strong>in</strong>g a calix[4]arene th<strong>in</strong> film. We have obta<strong>in</strong>edvery good response and high repeatability characteristics. Theadsorption-desorption k<strong>in</strong>etics are analyzed and discussed <strong>in</strong>details.f(Hz)0-2-4-6-8-10-12-1411%RH43%RH54%RH75%RH84%RH97%RH0 50 100 150 200 250 300 350time(s)Figure2 The frequency response curve QCM-based sensors tocyclic humidity change-f(Hz)642K'8.08k obs0.0455f( t)K(1ek a62.49( kobst)050 100 150 200time(s)k d0.0005)G (kJ/mol)Figure3 The experimental data (blue l<strong>in</strong>e) and the fit (red l<strong>in</strong>e)to the Langmuir adsorption isotherm equationIn summary, the QCM results show that calix[4]areneth<strong>in</strong> films are very sensitive to humidity and give reproducibleadsorption and desorption k<strong>in</strong>etic behavior to humiditychanges for short time periods. Our results open a new era tothe high-sensitivity and high-selectivity gas sensorapplications. This work was supported by TUBITAK underGrant No. TBAG- 109T240.-29.3*Correspond<strong>in</strong>g author: omermermer@gmail.com[1] H. Aizawa, et al., Sens. Actuators B 101 (2004) 150.[2] H. Zeng, et al., Sens. Actuators B 122 (2007) 1[3] Koshets I. A., et.al.,Sens. Actuators B 106, (2005), 177[4] Ohira, Sh<strong>in</strong>-I. , et.al., Talanta 2009, 77, 1814.[5] S. Okur,et.al., Talanta, Volume 81, Issues 1-2, 2010, Pages 248[6] D. S. Karpovich and G. J. Blanchard, Langmuir 1994,10, 3315[7] Y.L. Sun, et. al, Talanta 73, 857-861, 2007.[8] A. Erol, et.al., Sensors and Actuators B: Chem., 145, 2010, Pages 174-1806th Nanoscience and Nanotechnology Conference, zmir, 2010 677
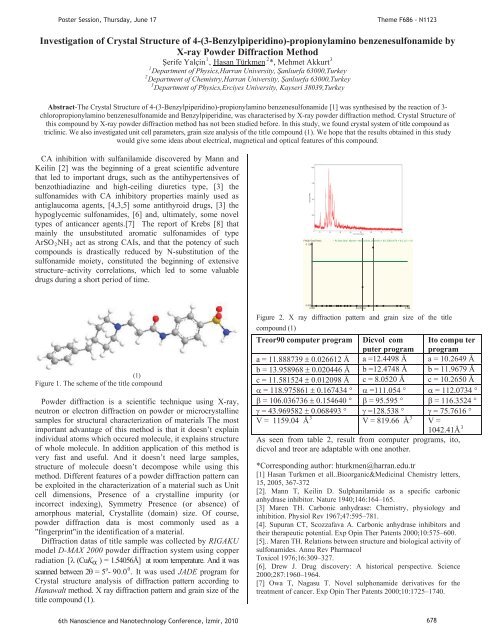
PPPoster Session, Thursday, June 17Theme F686 - N1123Investigation of Crystal Structure of 4-(3-Benzylpiperid<strong>in</strong>o)-propionylam<strong>in</strong>o benzenesulfonamide byX-ray Powder Diffraction Method123erife Yalç<strong>in</strong>P P, UHasan TürkmenUP P*, Mehmet AkkurtPPDepartment of Physics,Harran University, anlurfa 63000,TurkeyPDepartment of Chemistry,Harran University, anlurfa 63000,Turkey3PDepartment of Physics,Erciyes University, Kayseri 38039,TurkeyP21Abstract-The Crystal Structure of 4-(3-Benzylpiperid<strong>in</strong>o)-propionylam<strong>in</strong>o benzenesulfonamide [1] was synthesised by the reaction of 3-chloropropionylam<strong>in</strong>o benzenesulfonamide and Benzylpiperid<strong>in</strong>e, was characterised by X-ray powder diffraction method. Crystal Structure ofthis compound by X-ray powder diffraction method has not been studied before. In this study, we found crystal system of title compound astricl<strong>in</strong>ic. We also <strong>in</strong>vestigated unit cell parameters, gra<strong>in</strong> size analysis of the title compound (1). We hope that the results obta<strong>in</strong>ed <strong>in</strong> this studywould give some ideas about electrical, magnetical and optical features of this compound.CA <strong>in</strong>hibition with sulfanilamide discovered by Mann andKeil<strong>in</strong> [2] was the beg<strong>in</strong>n<strong>in</strong>g of a great scientific adventurethat led to important drugs, such as the antihypertensives ofbenzothiadiaz<strong>in</strong>e and high-ceil<strong>in</strong>g diuretics type, [3] thesulfonamides with CA <strong>in</strong>hibitory properties ma<strong>in</strong>ly used asantiglaucoma agents, [4,3,5] some antithyroid drugs, [3] thehypoglycemic sulfonamides, [6] and, ultimately, some noveltypes of anticancer agents.[7] The report of Krebs [8] thatma<strong>in</strong>ly the unsubstituted aromatic sulfonamides of typeArSOR2RNHR2R act as strong CAIs, and that the potency of suchcompounds is drastically reduced by N-substitution of thesulfonamide moiety, constituted the beg<strong>in</strong>n<strong>in</strong>g of extensivestructure–activity correlations, which led to some valuabledrugs dur<strong>in</strong>g a short period of time.FW(S)*Cos(Theta)0.128* Fit Size Only: XS(nm) = 86.6 (130.0), Stra<strong>in</strong>(%) = 0.0, ESD of Fit = 0.0, LC = 1.0(1)Figure 1. The scheme of the title compoundPowder diffraction is a scientific technique us<strong>in</strong>g X-ray,neutron or electron diffraction on powder or microcrystall<strong>in</strong>esamples for structural characterization of materials The mostimportant advantage of this method is that it doesn’t expla<strong>in</strong><strong>in</strong>dividual atoms which occured molecule, it expla<strong>in</strong>s structureof whole molecule. In addition application of this method isvery fast and useful. And it doesn’t need large samples,structure of molecule doesn’t decompose while us<strong>in</strong>g thismethod. Different features of a powder diffraction pattern canbe exploited <strong>in</strong> the characterization of a material such as Unitcell dimensions, Presence of a crystall<strong>in</strong>e impurity (or<strong>in</strong>correct <strong>in</strong>dex<strong>in</strong>g), Symmetry Presence (or absence) ofamorphous material, Crystallite (doma<strong>in</strong>) size. Of course,powder diffraction data is most commonly used as a"f<strong>in</strong>gerpr<strong>in</strong>t"<strong>in</strong> the identification of a material.Diffraction datas of title sample was collected by RIGAKUmodel D-MAX 2000 powder diffraction system us<strong>in</strong>g copperradiation [ (CuK ) = 1.54056Å] at room temperature. And it waso 0scanned between 2 = 5P P- 90.0 P P. It was used JADE program forCrystal structure analysis of diffraction pattern accord<strong>in</strong>g toHanawalt method. X ray diffraction pattern and gra<strong>in</strong> size of thetitle compound (1).0.0000.080 S<strong>in</strong>(Theta)0.396Figure 2. X ray diffraction pattern and gra<strong>in</strong> size of the titlecompound (1)Treor90 computer program Dicvol com Ito compu terputer program programa = 11.888739 0.026612 Å a =12.4498 Å a = 10.2649 Åb = 13.958968 0.020446 Å b =12.4748 Å b = 11.9679 Åc = 11.581524 0.012098 Å c = 8.0520 Å c = 10.2650 Å = 118.975861 0.167434 ° =111.054 ° = 112.0734 ° = 106.036736 0.154640 ° = 95.595 ° = 116.3524 ° = 43.969582 0.068493 ° =128.538 ° = 75.7616 °3V = 1159.04 ÅP3V = 819.66 ÅP V =1042.41ÅPAs seen from table 2, result from computer programs, ito,dicvol and treor are adaptable with one another.*Correspond<strong>in</strong>g author: hturkmen@harran.edu.tr[1] Hasan Turkmen et all..Bioorganic&Medic<strong>in</strong>al Chemistry letters,15, 2005, 367-372[2]. Mann T, Keil<strong>in</strong> D. Sulphanilamide as a specific carbonicanhydrase <strong>in</strong>hibitor. Nature 1940;146:164–165.[3] Maren TH. Carbonic anhydrase: Chemistry, physiology and<strong>in</strong>hibition. Physiol Rev 1967;47:595–781.[4]. Supuran CT, Scozzafava A. Carbonic anhydrase <strong>in</strong>hibitors andtheir therapeutic potential. Exp Op<strong>in</strong> Ther Patents 2000;10:575–600.[5].. Maren TH. Relations between structure and biological activity ofsulfonamides. Annu Rev PharmacolToxicol 1976;16:309–327.[6]. Drew J. Drug discovery: A historical perspective. Science2000;287:1960–1964.[7] Owa T, Nagasu T. Novel sulphonamide derivatives for thetreatment of cancer. Exp Op<strong>in</strong> Ther Patents 2000;10:1725–1740.36th Nanoscience and Nanotechnology Conference, zmir, 2010 678
- Page 1:
Poster Presentations3rd Day17 June
- Page 4 and 5:
Determination of Dielectric Anisotr
- Page 7 and 8:
Poster Session, Thursday, June 17Th
- Page 9 and 10:
PP mPP vs.P =P,PP (1)P andPoster Se
- Page 11 and 12:
PP mPP vs.P =P,PP (1)P andPoster Se
- Page 13 and 14:
PP andPoster Session, Thursday, Jun
- Page 15 and 16:
Poster Session, Thursday, June 17Th
- Page 17 and 18: PP and770 772 774 776 778 780 782 7
- Page 19 and 20: Poster Session, Thursday, June 17Th
- Page 21 and 22: Poster Session, Thursday, June 17Th
- Page 23 and 24: P25,Poster Session, Thursday, June
- Page 25 and 26: PP TOBBPoster Session, Thursday, Ju
- Page 27 and 28: PisPPisisisP,PisPoster Session, Thu
- Page 29 and 30: U NeslihanPPPPoster Session, Thursd
- Page 31 and 32: Poster Session, Thursday, June 17Th
- Page 33 and 34: PPPoster Session, Thursday, June 17
- Page 35 and 36: PPoster Session, Thursday, June 17T
- Page 37 and 38: P onP viaPP wereP upPoster Session,
- Page 39 and 40: P ·cm.PVPPPsPPPPP andPoster Sessio
- Page 41 and 42: Poster Session, Thursday, June 17Th
- Page 43 and 44: PPoster Session, Thursday, June 17T
- Page 45 and 46: PPoster Session, Thursday, June 17T
- Page 47 and 48: Poster Session, Thursday, June 17Th
- Page 49 and 50: PErkanPoster Session, Thursday, Jun
- Page 51 and 52: Poster Session, Thursday, June 17Th
- Page 53 and 54: Poster Session, Thursday, June 17Th
- Page 55 and 56: PPPP andPoster Session, Thursday, J
- Page 57 and 58: Poster Session, Thursday, June 17Th
- Page 59 and 60: Poster Session, Thursday, June 17Th
- Page 61 and 62: T PeptideTPP,PP,PP andTT2429TTTTTT
- Page 63 and 64: Poster Session, Thursday, June 17Th
- Page 65 and 66: PPoster Session, Thursday, June 17T
- Page 67: Poster Session, Thursday, June 17Th
- Page 71 and 72: Poster Session, Thursday, June 17Th
- Page 73 and 74: Poster Session, Thursday, June 17Th
- Page 75 and 76: PT AdditionalT ThePoster Session, T
- Page 77 and 78: Poster Session, Thursday, June 17Th
- Page 79 and 80: Poster Session, Thursday, June 17Th
- Page 81 and 82: Poster Session, Thursday, June 17Th
- Page 83 and 84: PPoster Session, Thursday, June 17T
- Page 85 and 86: Poster Session, Thursday, June 17Th
- Page 87 and 88: PPPoster Session, Thursday, June 17
- Page 89 and 90: Poster Session, Thursday, June 17Hu
- Page 91 and 92: Poster Session, Thursday, June 17Th
- Page 93 and 94: PPPPPPoster Session, Thursday, June
- Page 95 and 96: Poster Session, Thursday, June 17Th
- Page 97 and 98: Poster Session, Thursday, June 17Th
- Page 99 and 100: Poster Session, Thursday, June 17Th
- Page 101 and 102: PPoster Session, Thursday, June 17T
- Page 103 and 104: Poster Session, Thursday, June 17Th
- Page 105 and 106: PPPPPPPoster Session, Thursday, Jun
- Page 107 and 108: Poster Session, Thursday, June 17Th
- Page 109 and 110: PPPR2R PIN(80)PPgPP OzlemPPoster Se
- Page 111 and 112: Poster Session, Thursday, June 17Th
- Page 113 and 114: Poster Session, Thursday, June 17Th
- Page 115 and 116: P onPP toP coordinatedPPoster Sessi
- Page 117 and 118: PPPPP,PP,P(PR RmPoster Session, Thu
- Page 119 and 120:
Poster Session, Thursday, June 17Th
- Page 121 and 122:
Poster Session, Thursday, June 17Th
- Page 123 and 124:
PP InstitutePP DepartmentPoster Ses
- Page 125 and 126:
andPCPPoster Session, Thursday, Jun
- Page 127 and 128:
PP scatteringPYusufPP Corresponding
- Page 129 and 130:
PP toPoster Session, Thursday, June
- Page 131 and 132:
PP andPoster Session, Thursday, Jun
- Page 133 and 134:
PPPPoster Session, Thursday, June 1
- Page 135 and 136:
PPoster Session, Thursday, June 17T
- Page 137 and 138:
PPP andP (.cm).Poster Session, Thur
- Page 139 and 140:
PP tiltP andP editionPoster Session
- Page 141 and 142:
PP andPPoster Session, Thursday, Ju
- Page 143 and 144:
Poster Session, Thursday, June 17Th
- Page 145 and 146:
PP forP forP edit.PPoster Session,
- Page 147 and 148:
Poster Session, Thursday, June 17Th
- Page 149 and 150:
Poster Session, Thursday, June 17Th
- Page 151 and 152:
PP ionicPP ,PPoster Session, Thursd
- Page 153 and 154:
PP lightPoster Session, Thursday, J
- Page 155 and 156:
Poster Session, Thursday, June 17Th
- Page 157 and 158:
PPoster Session, Thursday, June 17T
- Page 159 and 160:
Poster Session, Thursday, June 17Th
- Page 161 and 162:
PandPoster Session, Thursday, June
- Page 163 and 164:
Poster Session, Thursday, June 17 T
- Page 165 and 166:
PPPoster Session, Thursday, June 17
- Page 167 and 168:
PPoster Session, Thursday, June 17T
- Page 169 and 170:
PPoster Session, Thursday, June 17T
- Page 171 and 172:
PPoster Session, Thursday, June 17T
- Page 173 and 174:
PP DepartmentNanoscienceTPPoster Se
- Page 175 and 176:
Poster Session, Thursday, June 17Th
- Page 177 and 178:
Poster Session, Thursday, June 17Th
- Page 179 and 180:
PPPoster Session, Thursday, June 17
- Page 181 and 182:
PPPPPoster Session, Thursday, June
- Page 183 and 184:
PPPPoster Session, Thursday, June 1
- Page 185 and 186:
PPoster Session, Thursday, June 17T
- Page 187 and 188:
PPoster Session, Thursday, June 17T
- Page 189 and 190:
PPoster Session, Thursday, June 17T
- Page 191 and 192:
Poster Session, Thursday, June 17Th
- Page 193 and 194:
Poster Session, Thursday, June 17Th
- Page 195 and 196:
0T0T0T0T AsPPPP werePoster Session,
- Page 197 and 198:
PPoster Session, Thursday, June 17T
- Page 199 and 200:
PPPPPoster Session, Thursday, June
- Page 201 and 202:
PPoster Session, Thursday, June 17T
- Page 203 and 204:
PPoster Session, Thursday, June 17T
- Page 205 and 206:
Poster Session, Thursday, June 17Th
- Page 207 and 208:
PPoster Session, Thursday, June 17T
- Page 209 and 210:
PPoster Session, Thursday, June 17T
- Page 211:
Poster Session, Thursday, June 17AF