- Page 2 and 3:
Biofuels Biofuels. Edited by Wim So
- Page 4 and 5:
Biofuels Edited by WIM SOETAERT Ghe
- Page 6 and 7:
Contents Series Preface ix Preface
- Page 8 and 9:
Contents vii 6.3 Biomass Gasificati
- Page 10 and 11:
Series Preface Renewable resources,
- Page 12 and 13:
Preface This volume on Biofuels fit
- Page 14 and 15:
Editors List of Contributors Wim So
- Page 16 and 17:
1 Biofuels in Perspective W. Soetae
- Page 18 and 19:
Table 1.1 Approximate average world
- Page 20 and 21:
Table 1.3 Energy yields of bio-ener
- Page 22 and 23:
Biofuels in Perspective 7 is burnt
- Page 24 and 25:
2 Sustainable Production of Cellulo
- Page 26 and 27:
Sustainable Production of Cellulosi
- Page 28 and 29:
Sustainable Production of Cellulosi
- Page 30 and 31:
Sustainable Production of Cellulosi
- Page 32 and 33:
Sustainable Production of Cellulosi
- Page 34 and 35:
Sustainable Production of Cellulosi
- Page 36 and 37:
Sustainable Production of Cellulosi
- Page 38 and 39:
Figure 2.9 2004 US adoption rates o
- Page 40 and 41:
Sustainable Production of Cellulosi
- Page 42 and 43:
Sustainable Production of Cellulosi
- Page 44 and 45:
Sustainable Production of Cellulosi
- Page 46 and 47:
Sustainable Production of Cellulosi
- Page 48 and 49:
Sustainable Production of Cellulosi
- Page 50 and 51:
Sustainable Production of Cellulosi
- Page 52 and 53:
Sustainable Production of Cellulosi
- Page 54 and 55:
3 Bio-Ethanol Development in the US
- Page 56 and 57:
Bio-Ethanol Development in the USA
- Page 58 and 59:
Biorefineries in Production (115) B
- Page 60 and 61:
Bio-Ethanol Development in the USA
- Page 62 and 63:
Bio-Ethanol Development in the USA
- Page 64 and 65:
Bio-Ethanol Development in the USA
- Page 66 and 67:
Cost of Cellulosic Ethanol, $ per g
- Page 68 and 69:
Bio-Ethanol Development in the USA
- Page 70 and 71:
4 Bio-Ethanol Development(s) in Bra
- Page 72 and 73:
Bio-Ethanol Development(s) in Brazi
- Page 74 and 75:
Share of energy consumption 100% 90
- Page 76 and 77:
Bio-Ethanol Development(s) in Brazi
- Page 78 and 79:
Bio-Ethanol Development(s) in Brazi
- Page 80 and 81:
Table 4.2 Main technological improv
- Page 82 and 83:
Bio-Ethanol Development(s) in Brazi
- Page 84 and 85:
4.7.4 Use of Fertilizers and Pestic
- Page 86 and 87:
Bio-Ethanol Development(s) in Brazi
- Page 88 and 89:
Bio-Ethanol Development(s) in Brazi
- Page 90:
Bio-Ethanol Development(s) in Brazi
- Page 93 and 94:
78 Biofuels Table 5.1 Biodiesel pro
- Page 95 and 96:
80 Biofuels CH 2 O CH O COR R1 + 3
- Page 97 and 98:
82 Biofuels short reaction times. 9
- Page 99 and 100:
84 Biofuels Table 5.4 Overview on h
- Page 101 and 102:
86 Biofuels Table 5.5 Critical cond
- Page 103 and 104:
88 Biofuels methoxide as catalyst u
- Page 105 and 106:
90 Biofuels KOH Methano Oil/Fat Aci
- Page 107 and 108:
92 Biofuels 16. J. Graille, P. Loza
- Page 110 and 111:
6 Bio-based Fischer-Tropsch Diesel
- Page 112 and 113:
6.2.1.2 Catalysts Bio-based Fischer
- Page 114 and 115:
Bio-based Fischer-Tropsch Diesel Pr
- Page 116 and 117:
Bio-based Fischer-Tropsch Diesel Pr
- Page 118 and 119:
Bio-based Fischer-Tropsch Diesel Pr
- Page 120 and 121:
Bio-based Fischer-Tropsch Diesel Pr
- Page 122 and 123:
Bio-based Fischer-Tropsch Diesel Pr
- Page 124 and 125:
Bio-based Fischer-Tropsch Diesel Pr
- Page 126 and 127:
Bio-based Fischer-Tropsch Diesel Pr
- Page 128 and 129:
Bio-based Fischer-Tropsch Diesel Pr
- Page 130 and 131:
Bio-based Fischer-Tropsch Diesel Pr
- Page 132 and 133:
7 Plant Oil Biofuel: Rationale, Pro
- Page 134 and 135:
Table 7.1 Market milestones for pla
- Page 136 and 137:
Water CO 2 Figure 7.1 Closed CO 2 l
- Page 138 and 139:
Plant Oil Biofuel: Rationale, Produ
- Page 140 and 141:
Plant Oil Biofuel: Rationale, Produ
- Page 142:
Plant Oil Biofuel: Rationale, Produ
- Page 145 and 146:
130 Biofuels Among the attractive f
- Page 147 and 148:
132 Biofuels Table 8.1 Enzymatic tr
- Page 149 and 150:
134 Biofuels conversion was maintai
- Page 151 and 152:
136 Biofuels (diglycerides) decreas
- Page 153 and 154:
138 Biofuels ME content (wt.%) Reac
- Page 155 and 156:
140 Biofuels Ratio of initial react
- Page 157 and 158:
142 Biofuels (a) 34 kDa 31 kDa 34 k
- Page 159 and 160:
144 Biofuels preparation of whole-c
- Page 161 and 162:
ability to reduce flocculation (% o
- Page 163 and 164:
148 Biofuels 5. R. C. Strayer, J. A
- Page 165 and 166:
150 Biofuels 46. H. Fukuda, Immobil
- Page 168 and 169: 9 Production of Biodiesel from Wast
- Page 170 and 171: Production of Biodiesel from Waste
- Page 172 and 173: Production of Biodiesel from Waste
- Page 174 and 175: 9.3.2 Processing of Crude and Waste
- Page 176 and 177: Heavy layer from alkaline neutralis
- Page 178 and 179: Production of Biodiesel from Waste
- Page 180 and 181: Production of Biodiesel from Waste
- Page 182 and 183: CPO I 1 1 Heating 60°C Reaction st
- Page 184 and 185: References Production of Biodiesel
- Page 186 and 187: 10 Biomass Digestion to Methane in
- Page 188 and 189: Cow manure Pig manure Yard manure B
- Page 190 and 191: Number of plants 3000 2500 2000 150
- Page 192 and 193: Biomass Digestion to Methane in Agr
- Page 194 and 195: Biomass Digestion to Methane in Agr
- Page 196 and 197: Table 10.4 Alternative forms of ene
- Page 198 and 199: Biomass Digestion to Methane in Agr
- Page 200 and 201: Wet fermentation 3 - 10% TS applica
- Page 202 and 203: Rel. Frequency [%] 35 30 25 20 15 1
- Page 204 and 205: Steam 2 1 Energy crops (& manure) D
- Page 206 and 207: Biomass Digestion to Methane in Agr
- Page 208 and 209: Biomass Digestion to Methane in Agr
- Page 210: Biomass Digestion to Methane in Agr
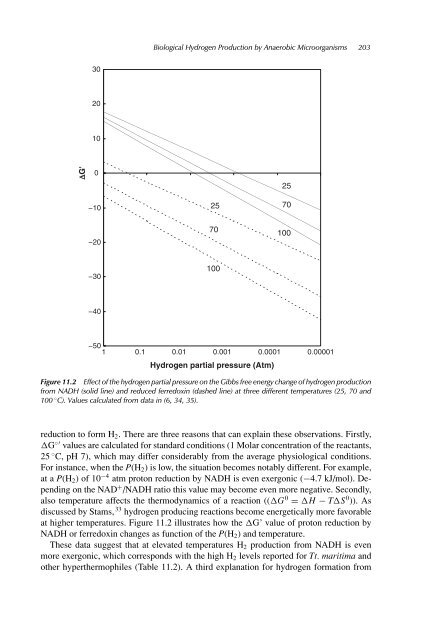
- Page 213 and 214: 198 Biofuels 11.1 Introduction Hydr
- Page 215 and 216: 200 Biofuels lactate 6 glucose 1 GA
- Page 217: Table 11.2 Continued Organism Domai
- Page 221 and 222: 206 Biofuels Clostridium thermocell
- Page 223 and 224: 208 Biofuels in particular in Cl. p
- Page 225 and 226: 210 Biofuels ferredoxin-dependent m
- Page 227 and 228: 212 Biofuels Concentration (mM) 45
- Page 229 and 230: 214 Biofuels genes of the glycolysi
- Page 231 and 232: 216 Biofuels 11. S. Tanisho and Y.
- Page 233 and 234: 218 Biofuels 49. M. J. Axley, D. A.
- Page 235 and 236: 220 Biofuels 83. P. J. Silva, E. C.
- Page 238 and 239: 12 Improving Sustainability of the
- Page 240 and 241: Table 12.1 Corn ethanol dry mill: e
- Page 242 and 243: Table 12.2 Atmospheric CO2 (equival
- Page 244 and 245: Table 12.3 Incremental CO2 equivale
- Page 246 and 247: Improving Sustainability of the Cor
- Page 248 and 249: Improving Sustainability of the Cor
- Page 250 and 251: Index italic entries indicate refer
- Page 252 and 253: iorefineries 3, 10, 11, 41 and corn
- Page 254 and 255: policy (official) 59-61 production
- Page 256 and 257: NADH 200-6, 207, 210 natural gas 1