198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
112 R.E. Melén<strong>de</strong>z · A.D. Hamilton<br />
structure of 15 to have expan<strong>de</strong>d their cavities from 11 Å (tak<strong>in</strong>g <strong>in</strong>to account<br />
van <strong>de</strong>r Waals radius) to 12.7 Å. In 16 the hexamer cavities are distorted and<br />
therefore the diameter is reduced to 10.4 Å (see Fig. 14) [49].<br />
3.2<br />
Ami<strong>de</strong>s<br />
A functional group that can parallel the hydrogen bond<strong>in</strong>g directionality of the<br />
carboxylic acid synthon is the ami<strong>de</strong> group . Primary and secondary ami<strong>de</strong>s<br />
[17], pyridones [50], and 2-am<strong>in</strong>opyrimid<strong>in</strong>es [51] form eight-membered r<strong>in</strong>g<br />
hydrogen bon<strong>de</strong>d dimers via the bi<strong>de</strong>ntate <strong>in</strong>teraction of the N-H and C=O<br />
groups. However, ami<strong>de</strong>s can also form l<strong>in</strong>ear cha<strong>in</strong>s through hydrogen bond<strong>in</strong>g<br />
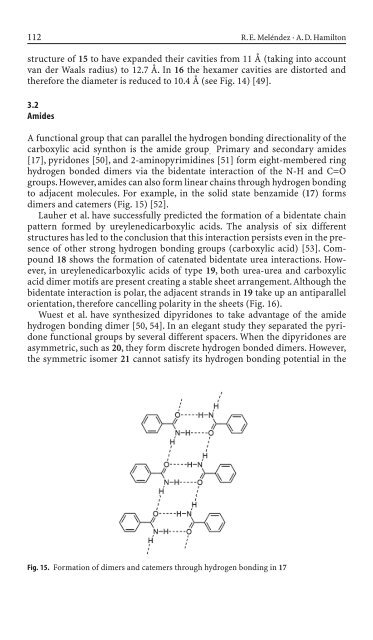
to adjacent molecules. For example, <strong>in</strong> the solid state benzami<strong>de</strong> (17) forms<br />
dimers and catemers (Fig. 15) [52].<br />
Lauher et al. have successfully predicted the formation of a bi<strong>de</strong>ntate cha<strong>in</strong><br />
pattern formed by ureylenedicarboxylic acids. The analysis of six different<br />
structures has led to the conclusion that this <strong>in</strong>teraction persists even <strong>in</strong> the presence<br />
of other strong hydrogen bond<strong>in</strong>g groups (carboxylic acid) [53]. Compound<br />
18 shows the formation of catenated bi<strong>de</strong>ntate urea <strong>in</strong>teractions. However,<br />
<strong>in</strong> ureylenedicarboxylic acids of type 19, both urea-urea and carboxylic<br />
acid dimer motifs are present creat<strong>in</strong>g a stable sheet arrangement. Although the<br />
bi<strong>de</strong>ntate <strong>in</strong>teraction is polar, the adjacent strands <strong>in</strong> 19 take up an antiparallel<br />
orientation, therefore cancell<strong>in</strong>g polarity <strong>in</strong> the sheets (Fig. 16).<br />
Wuest et al. have synthesized dipyridones to take advantage of the ami<strong>de</strong><br />
hydrogen bond<strong>in</strong>g dimer [50, 54]. In an elegant study they separated the pyridone<br />
functional groups by several different spacers. When the dipyridones are<br />
asymmetric, such as 20, they form discrete hydrogen bon<strong>de</strong>d dimers. However,<br />
the symmetric isomer 21 cannot satisfy its hydrogen bond<strong>in</strong>g potential <strong>in</strong> the<br />
Fig. 15. Formation of dimers and catemers through hydrogen bond<strong>in</strong>g <strong>in</strong> 17