198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
76 A. Nangia · G.R. Desiraju<br />
bonds <strong>in</strong> the three-po<strong>in</strong>t array with isolated hydrogen bonds; and lastly (4) to<br />
<strong>de</strong>monstrate the construction of supramolecular architectures us<strong>in</strong>g the synthon<br />
concept.<br />
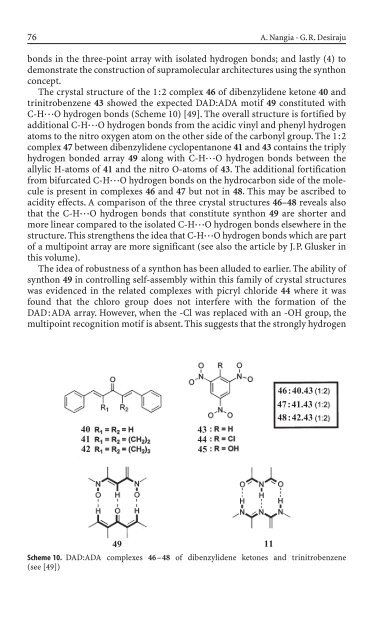
The crystal structure of the 1:2 complex 46 of dibenzyli<strong>de</strong>ne ketone 40 and<br />
tr<strong>in</strong>itrobenzene 43 showed the expected DAD:ADA motif 49 constituted with<br />
C-H◊◊◊O hydrogen bonds (Scheme 10) [49]. The overall structure is fortified by<br />
additional C-H◊◊◊O hydrogen bonds from the acidic v<strong>in</strong>yl and phenyl hydrogen<br />
atoms to the nitro oxygen atom on the other si<strong>de</strong> of the carbonyl group. The 1:2<br />
complex 47 between dibenzyli<strong>de</strong>ne cyclopentanone 41 and 43 conta<strong>in</strong>s the triply<br />
hydrogen bon<strong>de</strong>d array 49 along with C-H◊◊◊O hydrogen bonds between the<br />
allylic H-atoms of 41 and the nitro O-atoms of 43. The additional fortification<br />
from bifurcated C-H◊◊◊O hydrogen bonds on the hydrocarbon si<strong>de</strong> of the molecule<br />
is present <strong>in</strong> complexes 46 and 47 but not <strong>in</strong> 48. This may be ascribed to<br />
acidity effects. A comparison of the three crystal structures 46–48 reveals also<br />
that the C-H◊◊◊O hydrogen bonds that constitute synthon 49 are shorter and<br />
more l<strong>in</strong>ear compared to the isolated C-H◊◊◊O hydrogen bonds elsewhere <strong>in</strong> the<br />
structure. This strengthens the i<strong>de</strong>a that C-H◊◊◊O hydrogen bonds which are part<br />
of a multipo<strong>in</strong>t array are more significant (see also the article by J.P. Glusker <strong>in</strong><br />
this volume).<br />
The i<strong>de</strong>a of robustness of a synthon has been allu<strong>de</strong>d to earlier. The ability of<br />
synthon 49 <strong>in</strong> controll<strong>in</strong>g self-assembly with<strong>in</strong> this family of crystal structures<br />
was evi<strong>de</strong>nced <strong>in</strong> the related complexes with picryl chlori<strong>de</strong> 44 where it was<br />
found that the chloro group does not <strong>in</strong>terfere with the formation of the<br />
DAD:ADA array. However, when the -Cl was replaced with an -OH group, the<br />
multipo<strong>in</strong>t recognition motif is absent. This suggests that the strongly hydrogen<br />
40<br />
41<br />
42<br />
49<br />
43<br />
44<br />
45<br />
46:40.43<br />
47:41.43<br />
48:42.43<br />
Scheme 10. DAD:ADA complexes 46–48 of dibenzyli<strong>de</strong>ne ketones and tr<strong>in</strong>itrobenzene<br />
(see [49])<br />
11