198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
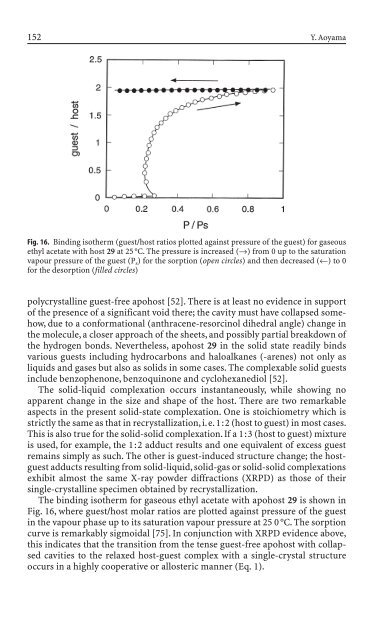
152 Y. Aoyama<br />
Fig. 16. B<strong>in</strong>d<strong>in</strong>g isotherm (guest/host ratios plotted aga<strong>in</strong>st pressure of the guest) for gaseous<br />
ethyl acetate with host 29 at 25 °C. The pressure is <strong>in</strong>creased (Æ) from 0 up to the saturation<br />
vapour pressure of the guest (P s) for the sorption (open circles) and then <strong>de</strong>creased (¨) to 0<br />
for the <strong>de</strong>sorption (filled circles)<br />
polycrystall<strong>in</strong>e guest-free apohost [52]. There is at least no evi<strong>de</strong>nce <strong>in</strong> support<br />
of the presence of a significant void there; the cavity must have collapsed somehow,<br />
due to a conformational (anthracene-resorc<strong>in</strong>ol dihedral angle) change <strong>in</strong><br />
the molecule, a closer approach of the sheets, and possibly partial breakdown of<br />
the hydrogen bonds. Nevertheless, apohost 29 <strong>in</strong> the solid state readily b<strong>in</strong>ds<br />
various guests <strong>in</strong>clud<strong>in</strong>g hydrocarbons and haloalkanes (-arenes) not only as<br />
liquids and gases but also as solids <strong>in</strong> some cases. The complexable solid guests<br />
<strong>in</strong>clu<strong>de</strong> benzophenone, benzoqu<strong>in</strong>one and cyclohexanediol [52].<br />
The solid-liquid complexation occurs <strong>in</strong>stantaneously, while show<strong>in</strong>g no<br />
apparent change <strong>in</strong> the size and shape of the host. There are two remarkable<br />
aspects <strong>in</strong> the present solid-state complexation. One is stoichiometry which is<br />
strictly the same as that <strong>in</strong> recrystallization, i.e. 1:2 (host to guest) <strong>in</strong> most cases.<br />
This is also true for the solid-solid complexation. If a 1:3 (host to guest) mixture<br />
is used, for example, the 1:2 adduct results and one equivalent of excess guest<br />
rema<strong>in</strong>s simply as such. The other is guest-<strong>in</strong>duced structure change; the hostguest<br />
adducts result<strong>in</strong>g from solid-liquid, solid-gas or solid-solid complexations<br />
exhibit almost the same X-ray pow<strong>de</strong>r diffractions (XRPD) as those of their<br />
s<strong>in</strong>gle-crystall<strong>in</strong>e specimen obta<strong>in</strong>ed by recrystallization.<br />
The b<strong>in</strong>d<strong>in</strong>g isotherm for gaseous ethyl acetate with apohost 29 is shown <strong>in</strong><br />
Fig. 16, where guest/host molar ratios are plotted aga<strong>in</strong>st pressure of the guest<br />
<strong>in</strong> the vapour phase up to its saturation vapour pressure at 25 0 °C. The sorption<br />
curve is remarkably sigmoidal [75]. In conjunction with XRPD evi<strong>de</strong>nce above,<br />
this <strong>in</strong>dicates that the transition from the tense guest-free apohost with collapsed<br />
cavities to the relaxed host-guest complex with a s<strong>in</strong>gle-crystal structure<br />
occurs <strong>in</strong> a highly cooperative or allosteric manner (Eq. 1).