198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
74 A. Nangia · G.R. Desiraju<br />
and robustness to the result<strong>in</strong>g supramolecular synthon. Multipo<strong>in</strong>t recognition<br />
between the DNA base pairs with Watson-Crick hydrogen bond<strong>in</strong>g schemes may<br />
be represented as DA:AD for a<strong>de</strong>n<strong>in</strong>e-thym<strong>in</strong>e and DDA:AAD for guan<strong>in</strong>ecytos<strong>in</strong>e<br />
(D=hydrogen bond donor, A=hydrogen bond acceptor). The dist<strong>in</strong>ct<br />
arrays DDA:AAD, DAD:ADA and DDD:AAA <strong>in</strong> three-po<strong>in</strong>t recognition motifs<br />
have been <strong>in</strong>vestigated separately by Zimmerman and Murray [39] and M<strong>in</strong>gos<br />
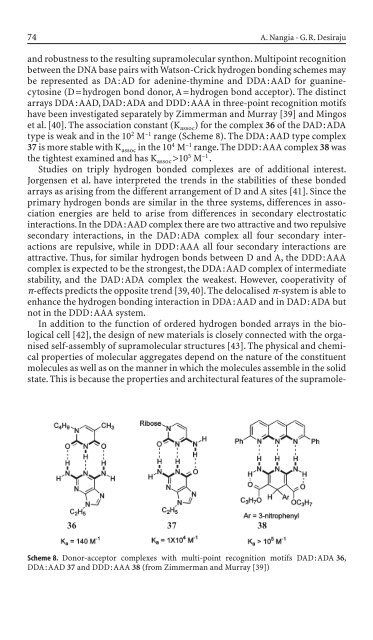
et al. [40]. The association constant (K assoc) for the complex 36 of the DAD : ADA<br />
type is weak and <strong>in</strong> the 10 2 M –1 range (Scheme 8). The DDA:AAD type complex<br />
37 is more stable with K assoc <strong>in</strong> the 10 4 M –1 range. The DDD:AAA complex 38 was<br />
the tightest exam<strong>in</strong>ed and has K assoc>10 5 M –1 .<br />
Studies on triply hydrogen bon<strong>de</strong>d complexes are of additional <strong>in</strong>terest.<br />
Jorgensen et al. have <strong>in</strong>terpreted the trends <strong>in</strong> the stabilities of these bon<strong>de</strong>d<br />
arrays as aris<strong>in</strong>g from the different arrangement of D and A sites [41]. S<strong>in</strong>ce the<br />
primary hydrogen bonds are similar <strong>in</strong> the three systems, differences <strong>in</strong> association<br />
energies are held to arise from differences <strong>in</strong> secondary electrostatic<br />
<strong>in</strong>teractions. In the DDA:AAD complex there are two attractive and two repulsive<br />
secondary <strong>in</strong>teractions, <strong>in</strong> the DAD:ADA complex all four secondary <strong>in</strong>teractions<br />
are repulsive, while <strong>in</strong> DDD:AAA all four secondary <strong>in</strong>teractions are<br />
attractive. Thus, for similar hydrogen bonds between D and A, the DDD:AAA<br />
complex is expected to be the strongest, the DDA:AAD complex of <strong>in</strong>termediate<br />
stability, and the DAD:ADA complex the weakest. However, cooperativity of<br />
p-effects predicts the opposite trend [39, 40]. The <strong>de</strong>localised p-system is able to<br />
enhance the hydrogen bond<strong>in</strong>g <strong>in</strong>teraction <strong>in</strong> DDA:AAD and <strong>in</strong> DAD:ADA but<br />
not <strong>in</strong> the DDD:AAA system.<br />
In addition to the function of or<strong>de</strong>red hydrogen bon<strong>de</strong>d arrays <strong>in</strong> the biological<br />
cell [42], the <strong>de</strong>sign of new materials is closely connected with the organised<br />
self-assembly of supramolecular structures [43]. The physical and chemical<br />
properties of molecular aggregates <strong>de</strong>pend on the nature of the constituent<br />
molecules as well as on the manner <strong>in</strong> which the molecules assemble <strong>in</strong> the solid<br />
state. This is because the properties and architectural features of the supramole-<br />
36 37 38<br />
Scheme 8. Donor-acceptor complexes with multi-po<strong>in</strong>t recognition motifs DAD:ADA 36,<br />
DDA:AAD 37 and DDD:AAA 38 (from Zimmerman and Murray [39])