198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Directional Aspects of Intermolecular Interactions 33<br />
found, but values may reach 12. Ionic radii vary with the coord<strong>in</strong>ation number<br />
[68]. For example, a cation with a tetrahedral coord<strong>in</strong>ation of four anions has<br />
93–95% the radius of the same cation with a coord<strong>in</strong>ation number of 6, but if<br />
the coord<strong>in</strong>ation number is 8, the radius is 103% that for coord<strong>in</strong>ation number<br />
6. Similarly, radii for the oxi<strong>de</strong> anion vary from 1.35 Å (coord<strong>in</strong>ation number 2),<br />
to 1.40 Å (coord<strong>in</strong>ation number 6), to 1.42 Å (coord<strong>in</strong>ation number 8).<br />
The directionality of metal-ion coord<strong>in</strong>ation can be consi<strong>de</strong>red <strong>in</strong> two ways –<br />
from the po<strong>in</strong>t of view of a coord<strong>in</strong>at<strong>in</strong>g ligand such as a carboxylate or imidazole<br />
group <strong>in</strong> a prote<strong>in</strong> si<strong>de</strong> cha<strong>in</strong>, and from the po<strong>in</strong>t of view of the metal ion,<br />
that is, how directional the bonds <strong>in</strong> its coord<strong>in</strong>ation sphere are. These two<br />
aspects of metal b<strong>in</strong>d<strong>in</strong>g are discussed here. They are relevant to the manner <strong>in</strong><br />
which metal ions mediate <strong>in</strong>termolecular as well as <strong>in</strong>tramolecular <strong>in</strong>teractions.<br />
6.1<br />
Metal Ion Coord<strong>in</strong>ation<br />
Metal ion coord<strong>in</strong>ation is generally consi<strong>de</strong>red to consist of anions arranged<br />
around the metal <strong>in</strong> or<strong>de</strong>r to fill space. Directional components of the <strong>in</strong>teractions<br />
<strong>in</strong>volved are not usually taken <strong>in</strong>to consi<strong>de</strong>ration. If, however, the cation<br />
has a rigid coord<strong>in</strong>ation sphere – a regular tetrahedron for coord<strong>in</strong>ation number<br />
4 or a regular octahedron for coord<strong>in</strong>ation number 6, and if at least two<br />
coord<strong>in</strong>ation positions are filled (as, for example, by a bi<strong>de</strong>ntate ligand) – then<br />
the directions of b<strong>in</strong>d<strong>in</strong>g of the other coord<strong>in</strong>ation positions are well fixed. Thus<br />
cation coord<strong>in</strong>ation can, <strong>in</strong> certa<strong>in</strong> cases, have a strong directional component.<br />
6.2<br />
Magnesium B<strong>in</strong>d<strong>in</strong>g<br />
Some metal ions have fairly rigid coord<strong>in</strong>ation spheres. A prime example is the<br />
divalent magnesium ion which shows a great preference for six oxygen atoms<br />
(such as from water or carboxylate groups) <strong>in</strong> its <strong>in</strong>ner coord<strong>in</strong>ation sphere [69].<br />
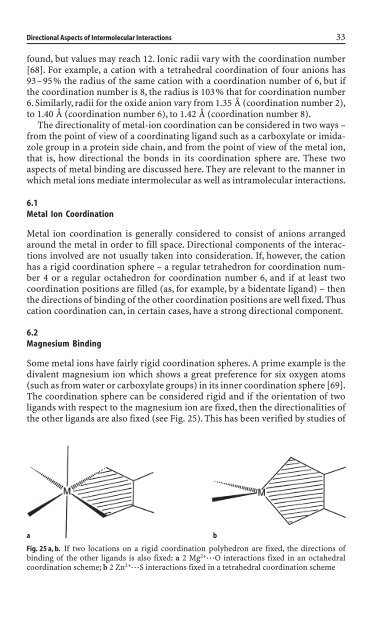
The coord<strong>in</strong>ation sphere can be consi<strong>de</strong>red rigid and if the orientation of two<br />
ligands with respect to the magnesium ion are fixed, then the directionalities of<br />
the other ligands are also fixed (see Fig. 25). This has been verified by studies of<br />
a b<br />
Fig. 25 a, b. If two locations on a rigid coord<strong>in</strong>ation polyhedron are fixed, the directions of<br />
b<strong>in</strong>d<strong>in</strong>g of the other ligands is also fixed: a 2 Mg 2+ ◊◊◊O <strong>in</strong>teractions fixed <strong>in</strong> an octahedral<br />
coord<strong>in</strong>ation scheme; b 2 Zn 2+ ◊◊◊S <strong>in</strong>teractions fixed <strong>in</strong> a tetrahedral coord<strong>in</strong>ation scheme