198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Hydrogen-Bon<strong>de</strong>d Ribbons, Tapes and Sheets as Motifs for Crystal Eng<strong>in</strong>eer<strong>in</strong>g 117<br />
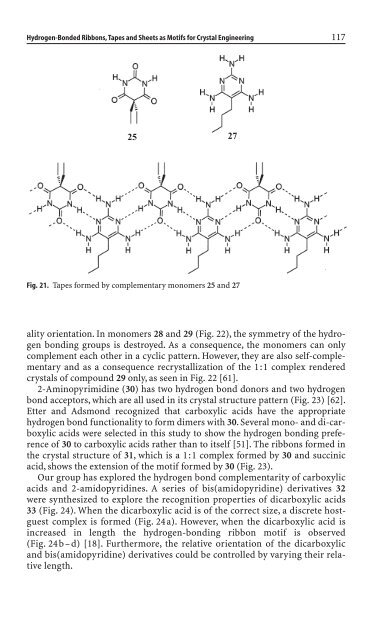
25<br />
Fig. 21. Tapes formed by complementary monomers 25 and 27<br />
ality orientation. In monomers 28 and 29 (Fig. 22), the symmetry of the hydrogen<br />
bond<strong>in</strong>g groups is <strong>de</strong>stroyed. As a consequence, the monomers can only<br />
complement each other <strong>in</strong> a cyclic pattern. However, they are also self-complementary<br />
and as a consequence recrystallization of the 1:1 complex ren<strong>de</strong>red<br />
crystals of compound 29 only, as seen <strong>in</strong> Fig. 22 [61].<br />
2-Am<strong>in</strong>opyrimid<strong>in</strong>e (30) has two hydrogen bond donors and two hydrogen<br />
bond acceptors, which are all used <strong>in</strong> its crystal structure pattern (Fig. 23) [62].<br />
Etter and Adsmond recognized that carboxylic acids have the appropriate<br />
hydrogen bond functionality to form dimers with 30. Several mono- and di-carboxylic<br />
acids were selected <strong>in</strong> this study to show the hydrogen bond<strong>in</strong>g preference<br />
of 30 to carboxylic acids rather than to itself [51]. The ribbons formed <strong>in</strong><br />
the crystal structure of 31, which is a 1:1 complex formed by 30 and succ<strong>in</strong>ic<br />
acid, shows the extension of the motif formed by 30 (Fig. 23).<br />
Our group has explored the hydrogen bond complementarity of carboxylic<br />
acids and 2-amidopyrid<strong>in</strong>es. A series of bis(amidopyrid<strong>in</strong>e) <strong>de</strong>rivatives 32<br />
were synthesized to explore the recognition properties of dicarboxylic acids<br />
33 (Fig. 24). When the dicarboxylic acid is of the correct size, a discrete hostguest<br />
complex is formed (Fig. 24a). However, when the dicarboxylic acid is<br />
<strong>in</strong>creased <strong>in</strong> length the hydrogen-bond<strong>in</strong>g ribbon motif is observed<br />
(Fig. 24b–d) [18]. Furthermore, the relative orientation of the dicarboxylic<br />
and bis(amidopyrid<strong>in</strong>e) <strong>de</strong>rivatives could be controlled by vary<strong>in</strong>g their relative<br />
length.<br />
27