198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
32 J.P. Glusker<br />
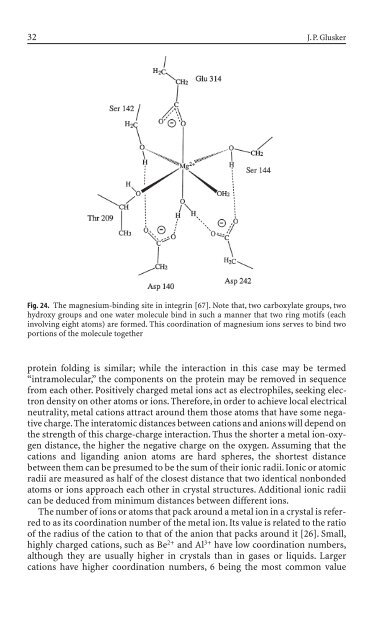
Fig. 24. The magnesium-b<strong>in</strong>d<strong>in</strong>g site <strong>in</strong> <strong>in</strong>tegr<strong>in</strong> [67]. Note that, two carboxylate groups, two<br />
hydroxy groups and one water molecule b<strong>in</strong>d <strong>in</strong> such a manner that two r<strong>in</strong>g motifs (each<br />
<strong>in</strong>volv<strong>in</strong>g eight atoms) are formed. This coord<strong>in</strong>ation of magnesium ions serves to b<strong>in</strong>d two<br />
portions of the molecule together<br />
prote<strong>in</strong> fold<strong>in</strong>g is similar; while the <strong>in</strong>teraction <strong>in</strong> this case may be termed<br />
“<strong>in</strong>tramolecular,” the components on the prote<strong>in</strong> may be removed <strong>in</strong> sequence<br />
from each other. Positively charged metal ions act as electrophiles, seek<strong>in</strong>g electron<br />
<strong>de</strong>nsity on other atoms or ions. Therefore, <strong>in</strong> or<strong>de</strong>r to achieve local electrical<br />
neutrality, metal cations attract around them those atoms that have some negative<br />
charge.The <strong>in</strong>teratomic distances between cations and anions will <strong>de</strong>pend on<br />
the strength of this charge-charge <strong>in</strong>teraction. Thus the shorter a metal ion-oxygen<br />
distance, the higher the negative charge on the oxygen. Assum<strong>in</strong>g that the<br />
cations and ligand<strong>in</strong>g anion atoms are hard spheres, the shortest distance<br />
between them can be presumed to be the sum of their ionic radii. Ionic or atomic<br />
radii are measured as half of the closest distance that two i<strong>de</strong>ntical nonbon<strong>de</strong>d<br />
atoms or ions approach each other <strong>in</strong> crystal structures. Additional ionic radii<br />
can be <strong>de</strong>duced from m<strong>in</strong>imum distances between different ions.<br />
The number of ions or atoms that pack around a metal ion <strong>in</strong> a crystal is referred<br />
to as its coord<strong>in</strong>ation number of the metal ion. Its value is related to the ratio<br />
of the radius of the cation to that of the anion that packs around it [26]. Small,<br />
highly charged cations, such as Be 2+ and Al 3+ have low coord<strong>in</strong>ation numbers,<br />
although they are usually higher <strong>in</strong> crystals than <strong>in</strong> gases or liquids. Larger<br />
cations have higher coord<strong>in</strong>ation numbers, 6 be<strong>in</strong>g the most common value