198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Directional Aspects of Intermolecular Interactions 31<br />
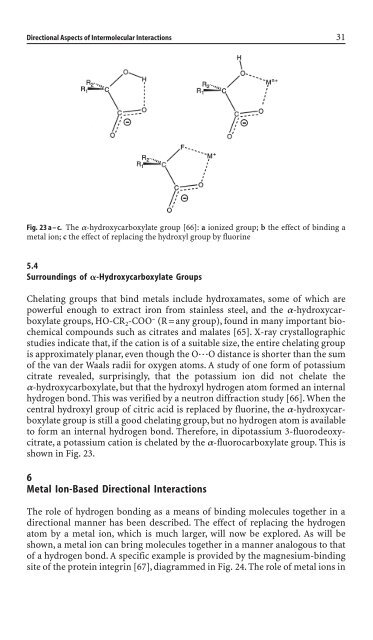
Fig. 23 a – c. The a-hydroxycarboxylate group [66]: a ionized group; b the effect of b<strong>in</strong>d<strong>in</strong>g a<br />
metal ion; c the effect of replac<strong>in</strong>g the hydroxyl group by fluor<strong>in</strong>e<br />
5.4<br />
Surround<strong>in</strong>gs of -Hydroxycarboxylate Groups<br />
Chelat<strong>in</strong>g groups that b<strong>in</strong>d metals <strong>in</strong>clu<strong>de</strong> hydroxamates, some of which are<br />
powerful enough to extract iron from sta<strong>in</strong>less steel, and the a-hydroxycarboxylate<br />
groups, HO-CR 2-COO – (R=any group), found <strong>in</strong> many important biochemical<br />
compounds such as citrates and malates [65]. X-ray crystallographic<br />
studies <strong>in</strong>dicate that, if the cation is of a suitable size, the entire chelat<strong>in</strong>g group<br />
is approximately planar, even though the O◊◊◊O distance is shorter than the sum<br />
of the van <strong>de</strong>r Waals radii for oxygen atoms. A study of one form of potassium<br />
citrate revealed, surpris<strong>in</strong>gly, that the potassium ion did not chelate the<br />
a-hydroxycarboxylate, but that the hydroxyl hydrogen atom formed an <strong>in</strong>ternal<br />
hydrogen bond. This was verified by a neutron diffraction study [66]. When the<br />
central hydroxyl group of citric acid is replaced by fluor<strong>in</strong>e, the a-hydroxycarboxylate<br />
group is still a good chelat<strong>in</strong>g group, but no hydrogen atom is available<br />
to form an <strong>in</strong>ternal hydrogen bond. Therefore, <strong>in</strong> dipotassium 3-fluoro<strong>de</strong>oxycitrate,<br />
a potassium cation is chelated by the a-fluorocarboxylate group. This is<br />
shown <strong>in</strong> Fig. 23.<br />
6<br />
Metal Ion-Based Directional Interactions<br />
The role of hydrogen bond<strong>in</strong>g as a means of b<strong>in</strong>d<strong>in</strong>g molecules together <strong>in</strong> a<br />
directional manner has been <strong>de</strong>scribed. The effect of replac<strong>in</strong>g the hydrogen<br />
atom by a metal ion, which is much larger, will now be explored. As will be<br />
shown, a metal ion can br<strong>in</strong>g molecules together <strong>in</strong> a manner analogous to that<br />
of a hydrogen bond. A specific example is provi<strong>de</strong>d by the magnesium-b<strong>in</strong>d<strong>in</strong>g<br />
site of the prote<strong>in</strong> <strong>in</strong>tegr<strong>in</strong> [67], diagrammed <strong>in</strong> Fig. 24. The role of metal ions <strong>in</strong>