198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
64 A. Nangia · G.R. Desiraju<br />
[23] <strong>in</strong> 1973 and 1977 represented the most complex natural product synthesised<br />
at that time. The project spanned two cont<strong>in</strong>ents and occupied an army of<br />
researchers dur<strong>in</strong>g a period of 12 years. The second “chemical Everest” of molecular<br />
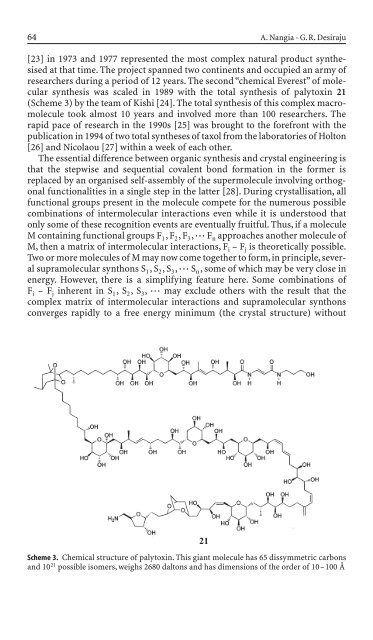
synthesis was scaled <strong>in</strong> <strong>198</strong>9 with the total synthesis of palytox<strong>in</strong> 21<br />
(Scheme 3) by the team of Kishi [24]. The total synthesis of this complex macromolecule<br />
took almost 10 years and <strong>in</strong>volved more than 100 researchers. The<br />
rapid pace of research <strong>in</strong> the 1990s [25] was brought to the forefront with the<br />
publication <strong>in</strong> 1994 of two total syntheses of taxol from the laboratories of Holton<br />
[26] and Nicolaou [27] with<strong>in</strong> a week of each other.<br />
The essential difference between organic synthesis and crystal eng<strong>in</strong>eer<strong>in</strong>g is<br />
that the stepwise and sequential covalent bond formation <strong>in</strong> the former is<br />
replaced by an organised self-assembly of the supermolecule <strong>in</strong>volv<strong>in</strong>g orthogonal<br />
functionalities <strong>in</strong> a s<strong>in</strong>gle step <strong>in</strong> the latter [28]. Dur<strong>in</strong>g crystallisation, all<br />
functional groups present <strong>in</strong> the molecule compete for the numerous possible<br />
comb<strong>in</strong>ations of <strong>in</strong>termolecular <strong>in</strong>teractions even while it is un<strong>de</strong>rstood that<br />
only some of these recognition events are eventually fruitful. Thus, if a molecule<br />
M conta<strong>in</strong><strong>in</strong>g functional groups F 1,F 2,F 3, ◊◊◊ F n approaches another molecule of<br />
M, then a matrix of <strong>in</strong>termolecular <strong>in</strong>teractions, F i – F j is theoretically possible.<br />
Two or more molecules of M may now come together to form,<strong>in</strong> pr<strong>in</strong>ciple,several<br />
supramolecular synthons S 1,S 2,S 3, ◊◊◊ S n, some of which may be very close <strong>in</strong><br />
energy. However, there is a simplify<strong>in</strong>g feature here. Some comb<strong>in</strong>ations of<br />
F i – F j <strong>in</strong>herent <strong>in</strong> S 1,S 2,S 3, ◊◊◊ may exclu<strong>de</strong> others with the result that the<br />
complex matrix of <strong>in</strong>termolecular <strong>in</strong>teractions and supramolecular synthons<br />
converges rapidly to a free energy m<strong>in</strong>imum (the crystal structure) without<br />
Scheme 3. Chemical structure of palytox<strong>in</strong>. This giant molecule has 65 dissymmetric carbons<br />
and 10 21 possible isomers, weighs 2680 daltons and has dimensions of the or<strong>de</strong>r of 10–100 Å<br />
21