198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
44 J.P. Glusker<br />
bond<strong>in</strong>g capabilities of the two compounds are different. Methotrexate is found<br />
<strong>in</strong> a cavity 15 Å <strong>de</strong>ep <strong>in</strong> the E. coli enzyme complex. The entire <strong>in</strong>hibitor molecule<br />
is bound by a system of hydrogen bond and hydrophobic <strong>in</strong>teractions. A<br />
similar b<strong>in</strong>d<strong>in</strong>g is found for the E. coli and L. casei enzyme complexes.<br />
The enzyme promotes hydri<strong>de</strong> transfer from NADPH to the pter<strong>in</strong> r<strong>in</strong>g. This<br />
is facilitated by the <strong>de</strong>velopment of a charge on C6 and by protonation of N5. The<br />
absolute configuration of tetrahydrofolate has been established [89, 90] to be S<br />
at C6, but if dihydrofolate is bound <strong>in</strong> the same orientation as methotrexate, the<br />
configuration should be R, as shown <strong>in</strong> Fig. 35. When dihydrofolate (or tetrahydrofolate)<br />
is bound <strong>in</strong> the enzyme <strong>in</strong> the same way as methotrexate, the hydrogen<br />
atom that is transferred to NADPH is po<strong>in</strong>t<strong>in</strong>g <strong>in</strong> the wrong direction. Thus<br />
dihydrofolate and methotrexate must b<strong>in</strong>d to the enzyme with different orientations<br />
as established later by X-ray crystallographic studies. Studies of additional<br />
bound substrate analogues and <strong>in</strong>hibitors [91, 92] confirm that b<strong>in</strong>d<strong>in</strong>g <strong>in</strong><br />
the active site of the enzyme is governed ma<strong>in</strong>ly by hydrogen bond<strong>in</strong>g, and not<br />
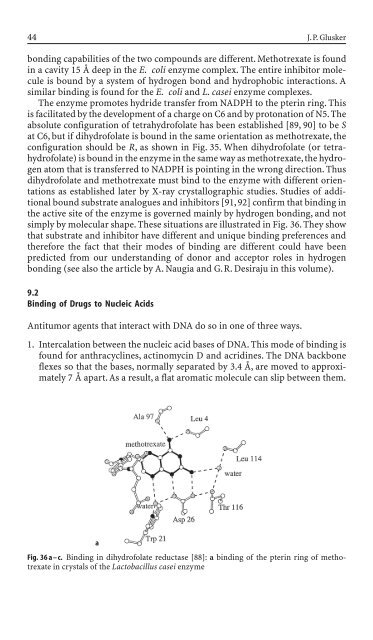
simply by molecular shape. These situations are illustrated <strong>in</strong> Fig. 36. They show<br />
that substrate and <strong>in</strong>hibitor have different and unique b<strong>in</strong>d<strong>in</strong>g preferences and<br />
therefore the fact that their mo<strong>de</strong>s of b<strong>in</strong>d<strong>in</strong>g are different could have been<br />
predicted from our un<strong>de</strong>rstand<strong>in</strong>g of donor and acceptor roles <strong>in</strong> hydrogen<br />
bond<strong>in</strong>g (see also the article by A. Naugia and G.R. Desiraju <strong>in</strong> this volume).<br />
9.2<br />
B<strong>in</strong>d<strong>in</strong>g of Drugs to Nucleic Acids<br />
Antitumor agents that <strong>in</strong>teract with DNA do so <strong>in</strong> one of three ways.<br />
1. Intercalation between the nucleic acid bases of DNA. This mo<strong>de</strong> of b<strong>in</strong>d<strong>in</strong>g is<br />
found for anthracycl<strong>in</strong>es, act<strong>in</strong>omyc<strong>in</strong> D and acrid<strong>in</strong>es. The DNA backbone<br />
flexes so that the bases, normally separated by 3.4 Å, are moved to approximately<br />
7 Å apart. As a result, a flat aromatic molecule can slip between them.<br />
a<br />
Fig. 36 a – c. B<strong>in</strong>d<strong>in</strong>g <strong>in</strong> dihydrofolate reductase [88]: a b<strong>in</strong>d<strong>in</strong>g of the pter<strong>in</strong> r<strong>in</strong>g of methotrexate<br />
<strong>in</strong> crystals of the Lactobacillus casei enzyme