198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
46 J.P. Glusker<br />
2. Non-<strong>in</strong>tercalative groove b<strong>in</strong>d<strong>in</strong>g. The drug lies <strong>in</strong> one of the grooves <strong>in</strong> DNA,<br />
follow<strong>in</strong>g the helical contour of DNA as it b<strong>in</strong>ds. Netrops<strong>in</strong> and distamyc<strong>in</strong><br />
b<strong>in</strong>d <strong>in</strong> this way.<br />
3. Covalent bond formation. This occurs with mitomyc<strong>in</strong> C.<br />
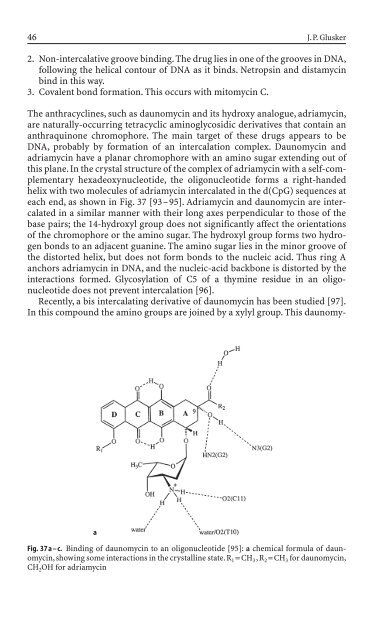
The anthracycl<strong>in</strong>es, such as daunomyc<strong>in</strong> and its hydroxy analogue, adriamyc<strong>in</strong>,<br />
are naturally-occurr<strong>in</strong>g tetracyclic am<strong>in</strong>oglycosidic <strong>de</strong>rivatives that conta<strong>in</strong> an<br />
anthraqu<strong>in</strong>one chromophore. The ma<strong>in</strong> target of these drugs appears to be<br />
DNA, probably by formation of an <strong>in</strong>tercalation complex. Daunomyc<strong>in</strong> and<br />
adriamyc<strong>in</strong> have a planar chromophore with an am<strong>in</strong>o sugar extend<strong>in</strong>g out of<br />
this plane. In the crystal structure of the complex of adriamyc<strong>in</strong> with a self-complementary<br />
hexa<strong>de</strong>oxynucleoti<strong>de</strong>, the oligonucleoti<strong>de</strong> forms a right-han<strong>de</strong>d<br />
helix with two molecules of adriamyc<strong>in</strong> <strong>in</strong>tercalated <strong>in</strong> the d(CpG) sequences at<br />
each end, as shown <strong>in</strong> Fig. 37 [93–95]. Adriamyc<strong>in</strong> and daunomyc<strong>in</strong> are <strong>in</strong>tercalated<br />
<strong>in</strong> a similar manner with their long axes perpendicular to those of the<br />
base pairs; the 14-hydroxyl group does not significantly affect the orientations<br />
of the chromophore or the am<strong>in</strong>o sugar. The hydroxyl group forms two hydrogen<br />
bonds to an adjacent guan<strong>in</strong>e. The am<strong>in</strong>o sugar lies <strong>in</strong> the m<strong>in</strong>or groove of<br />
the distorted helix, but does not form bonds to the nucleic acid. Thus r<strong>in</strong>g A<br />
anchors adriamyc<strong>in</strong> <strong>in</strong> DNA, and the nucleic-acid backbone is distorted by the<br />
<strong>in</strong>teractions formed. Glycosylation of C5 of a thym<strong>in</strong>e residue <strong>in</strong> an oligonucleoti<strong>de</strong><br />
does not prevent <strong>in</strong>tercalation [96].<br />
Recently, a bis <strong>in</strong>tercalat<strong>in</strong>g <strong>de</strong>rivative of daunomyc<strong>in</strong> has been studied [97].<br />
In this compound the am<strong>in</strong>o groups are jo<strong>in</strong>ed by a xylyl group. This daunomy-<br />
a<br />
Fig. 37 a – c. B<strong>in</strong>d<strong>in</strong>g of daunomyc<strong>in</strong> to an oligonucleoti<strong>de</strong> [95]: a chemical formula of daunomyc<strong>in</strong>,<br />
show<strong>in</strong>g some <strong>in</strong>teractions <strong>in</strong> the crystall<strong>in</strong>e state. R 1=CH 3,R 2=CH 3 for daunomyc<strong>in</strong>,<br />
CH 2OH for adriamyc<strong>in</strong>